* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2/a
Quantum field theory wikipedia , lookup
Nuclear structure wikipedia , lookup
Measurement in quantum mechanics wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Coherent states wikipedia , lookup
Future Circular Collider wikipedia , lookup
Quantum entanglement wikipedia , lookup
ATLAS experiment wikipedia , lookup
Bell's theorem wikipedia , lookup
History of quantum field theory wikipedia , lookup
Monte Carlo methods for electron transport wikipedia , lookup
Renormalization group wikipedia , lookup
Quantum chaos wikipedia , lookup
Quantum mechanics wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Renormalization wikipedia , lookup
Elementary particle wikipedia , lookup
Matrix mechanics wikipedia , lookup
Wave function wikipedia , lookup
Electron scattering wikipedia , lookup
Identical particles wikipedia , lookup
Quantum potential wikipedia , lookup
Quantum vacuum thruster wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Quantum state wikipedia , lookup
Probability amplitude wikipedia , lookup
Wave packet wikipedia , lookup
Path integral formulation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Relational approach to quantum physics wikipedia , lookup
Double-slit experiment wikipedia , lookup
EPR paradox wikipedia , lookup
Quantum logic wikipedia , lookup
Photon polarization wikipedia , lookup
Quantum tunnelling wikipedia , lookup
Canonical quantization wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Hidden variable theory wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Old quantum theory wikipedia , lookup
Uncertainty principle wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
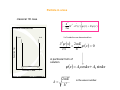
Introduction to Quantum Properties of Solid State Devices Principles of Quantum Mechanics 1) The principle of Energy Quanta: Experiments which showed inconsistency between experimental results and classical theories: Principles of Quantum Mechanics Thermal Radiation • Classical Physics: the frequency of the radiation of a heated cavity should increase monotonically as the thermal energy of the cavity increases. • Observations: the frequency of the radiation of a heated cavity increases only up to a certain energy then decreases. Principles of Quantum Mechanics When a particle jumps from one discrete state to another, it either absorbs or emits energy quanta (most frequently it is photon) which corresponds to a particular wavelength associated with the difference of the two states’ energy Max Planck’s postulate (1900): explains blackbody radiation E = hυ Principles of Quantum Mechanics Hydrogen Atoms: • Classical Physics: The atomic spectra should be continuous. • Observations: The atomic spectra contain only some discrete lines. Photoelectric effect: • Classical Physics: The absorption of optical energy by the electrons in a metal is independent of light frequency. • Observations: The absorption of optical energy by the electrons in a metal depends on optical frequency. Principles of Quantum Mechanics • Planck’s postulate in 1900 that radiation from a heated sample is emitted in discrete units of energy, called quanta. E =hυ h is the Planck’s constant, υ is frequency of the radiation Principles of Quantum Mechanics • Photoelectric Effect • Einstein in 1905 took a step further to interpret the photoelectric results by suggesting that the electromagnetic radiation exists in the form of packets of energy. This particle-like packet of energy is called photon. Photoelectric Effect KE = 1 2 mv = hν − hν 0 = hν − Φ 2 Principles of Quantum Mechanics 2) Wave-Particle Duality Principle • • • • Photoelectric Effect (particle) Compton Effect (particle) Diffraction pattern by electrons (wave) Since waves behave as particles, then particles should be expected to show wave-like properties. • de Broglie hypothesized that the wavelength of a particle can be expressed as λ=h/p • The momentum of a photon is then: p = h/λ =ħk Principles of Quantum Mechanics 3) The Uncertainty Principle (Heisenberg) • First statement: • It is impossible to simultaneously describe with absolute accuracy the position and momentum of a particle. • Δx Δp ≥ ħ • ħ is very small so this principle only applies to very small particles • Second statement: • It is impossible to simultaneously describe with absolute accuracy the energy of a particle and the instant of time the particle has this energy. • ΔE Δt ≥ ħ A Particle in a Box Classical Mechanics vs. Quantum Mechanics • CM: The particle remains trapped inside the box and can acquire any energy available to it . • QM: A particle trapped in such a box cannot have any energy, and will have only certain allowed energy. • CM: If no other forces are in the system, then the particle should have Brownian motion and has an equal probability of being anywhere and everywhere in the box. • QM: The particle has certain preferable points, where the probability of finding the particle is more than in other parts. • CM: The exact position and momentum of the particle should be obtainable simultaneously for the particle in question. • QM: We cannot measure the momentum and the position of the particle in the box simultaneously without some uncertainty. A Particle in a Box Classical Mechanics vs. Quantum Mechanics • CM: At a particular moment, the exact energy of the particle should be measurable. • QM: At any instant of time, the energy is not measurable above a certain extent. • CM: The particle must be confined in the box and has no chance of escaping the box as long as its energy is lower than that of the box. • QM: The particle has a finite chance of escaping the system even with energy lower than the box. • CM: If by any chance the particle attains energy larger than that of the box, the particle will certainly leave the box. • QM: If by any chance the particle attains energy larger than that of the box, there remains certain probability that particle might still be trapped inside. Principle of Quantum Mechanics Basic Postulates – Late 1920’s Ervin Schrodinger developed wave mechanics based on Planck’s theory and de Broglie’s idea of the wave nature of matter. – Warner Heisenberg formulated an alternative approach in terms of matrix algebra called Matrix Mechanics. Principle of Quantum Mechanics Basic Postulates • Dynamical Variables in Physics: position, momentum, energy • In classical mechanics the state of a system with a number of particles at any time is defined by designating the particle and momentum coordinates of all particles. • In quantum mechanics the state of a system is defined by a state function Ψ that contains all the information we can obtain about the system. • To approach quantum mechanics we consider several postulates that are assumed to be true Principle of Quantum Mechanics Basic Postulates • Postulate I: – There exists a state function Ψ(r;t) which contains all the measurable information about each particle of a physical system. Ψ(r;t) is also called wave function. • Postulate II: – Every dynamical variable has a corresponding operator. This operator is operated on the state function to obtain measurable information about the system. Principle of Quantum Mechanics Basic Postulates In a one-dimensional system Dynamical Variables: • Position r • Momentum p • Total Energy E • Potential Energy V(r) Principle of Quantum Mechanics Basic Postulates Dynamical Variable (1-D & 3-D) Position x , r Momentum p (x; r) Total Energy E Quantum Mechanic Operator ∧ X ≡ X, ∧ r≡r h ∂ Pˆx ≡ J ∂X ∧ Pr ≡ h ∂ j ∂r h ∂ Eˆ ≡ − J ∂t Potential Energy V(x; r) Vˆ ( x ; r ) ≡ V ( x ; r ) Principle of Quantum Mechanics Basic Postulates • Postulate III: – The state function Ψ(r;t) and its space derivative ∂Ψ/∂r must be continuous finite and single-valued for all values of r. • Postulate IV The state function must be normalized i.e ∞ ∗ ΨΨ dr = 1 ∫ −∞ – Ψ* is the complex conjugate of Ψ. Obviously Ψ Ψ* is a positive and real number and is equal to /Ψ/². Principle of Quantum Mechanics Basic Postulates • Assume a single particle-like electron; then Ψ Ψ* is interpreted as the statistical probability that the particle is found in a distance element dx at any instant of time. Thus Ψ Ψ* represents the probability density. Principle of Quantum Mechanics Basic Postulates Postulate V. • The average value 〈Q〉 of any variable corresponding to the state function Ψ is given by expectation value: ∞ Q = ∫Ψ ∗ ∧ Q Ψ dr −∞ • • 〈Q〉 is the expected value of any observation. Once the state function corresponding to any particle was found, it is possible to calculate the average position, energy and momentum of the particle within the limit of the uncertainty principle. Principle of Quantum Mechanics Schrodinger Equations Assume: p2 E= + V ( x, y, z ) 2m where p 2 = p x2 + p 2y + p z2 Recall the operators were defined in postulate II; p2 − h2 2 ≡ ∇ 2m 2m ∧ V ( x, y, z) ≡ V ( x, y, z) h ∂ E≡− j ∂t Schrodinger Equations: ⎛ h2 2 ⎞ ∂ ∇ + V (r )⎟⎟ψ (r , t ) jh ψ (r , t ) = ⎜⎜ − ∂t ⎝ 2m ⎠ The potential energy term forms the necessary link between quantum mechanics and the real world. It contains all environmental factors that could influence the state of a particle. As an example for a particle in a deep potential well: En 2 n 2π 2 h = 2 ma 2 2 ψn = sin Kx a Note than p(r)= ΨΨ* = ψψ* i.e. probability density is time -independent and define an stationary state Time dependant Schrödinger’s Equation ∂ j h ψ (r , t ) = ∂t single-particle 3D time-dependent Schrödinger equation Let’s assume : ψ (r , t ) = ψ (r )ψ (t ) Divide both sides by ψ (r )ψ (t ) Time –dependent part: jh dψ (t) =E ψ (t) dt 1 dψ (t) jE → =− ψ (t) dt h ψ (t ) = e − jEt / h ⎛ h2 2 ∧ ⎞ ⎜⎜ − ∇ + V (r )⎟⎟ψ (r , t ) 2 m ⎠ ⎝ ⎡ h2 2 ∧ ⎤ d ψ (t ) ψ ( r ) jh = ψ (t ) ⎢ − ∇ + V ( r ) ⎥ψ ( r ) dt ⎣ 2m ⎦ ∧ ⎤ jh d ψ (t ) 1 ⎡ h2 2 = − ∇ + V ( r ) ⎥ψ ( r ) ψ ( t ) dt ψ ( r ) ⎢⎣ 2 m ⎦ Time-independent part: And General solution: ⎡ h2 2 ∧ ⎤ ∇ + V ( r ) ⎥ψ ( r ) = Eψ ( r ) ⎢− ⎣ 2m ⎦ ψ ( r , t ) = e − jEt / hψ ( r ) Some Remarks 2 ψ ( r , t ) = ψ ∗ ( r , t )ψ ( r , t ) = e − jEt / hψ ( r ) ∗ e − jEt / hψ ( r ) = ψ ( r ) ∗ψ ( r ) Probability density is time -independent and define an stationary state. < A >= ∫ψ ∗ ( r , t ) Aψ ( r , t ) = ∫ψ ∗ ( r )ψ (r ) The expectation value for any time-independent operator is also time-independent plane wave If there is no force acting on the particle, then the potential function V(r) will be constant and we must have E>V(r). assume ψ (t ) = e V(r)=0 − j ( E h )t ψ ( x ) = Ae in +x direction ∂ 2ψ ( x ) 2mE + 2 ψ (x ) = 0 2 h ∂x time dependant part ( ) ⎛ j ⎞ ⎜ x 2 mE − Et ⎟ ⎝h ⎠ ψ (x ) = ψ ( x ) = Ae( jx + Be Ae ( ) ⎛ j ⎞ ⎜ − x 2 mE + Et ⎟ ⎝ h ⎠ j ( kx − ω t ) 2 mE h ) + Be(− jx 2 mE h ) k=2π/λ or λ=h/√(2mE) λ=h/p The probability density function ψ(x,t)ψ*(x,t)=AA* a free particle with a well defined energy will also have a well defined wavelength and momentum. is a constant independent of position. a free particle with a well defined momentum can be found anywhere with equal probability. Particle in a box classical 1D case ⎡ h2 2 ∧ ⎤ ∇ + V (r )⎥ψ (r ) = Eψ (r ) ⎢− ⎦ ⎣ 2m 1D Infinite Quantum Well U=8 U=8 V=0 inside the one dimensional box Function f(x) ∂ 2ψ ( x ) 2mE + 2 ψ (x ) = 0 2 ∂x h A particular form of solution X=1 X=0 U=0 Discrete X axis ψ (x) = A1 coskx + A2 sinkx X=n X=n+1 k= 2mE h2 is the wave number. Particle in a box… ψ (x ) = A2 sin kx First boundary condition, x=0, ψ(0)=0 then A1=0 second boundary condition ψ (a ) = A2 sin kx = 0 x=a, ψ(a)=0 a from the normalization condition ∫ (A 2 2 ) sin 2 kx dx = 1 k=nπ/a A2=√(2/a) 0 Final solution ψ (x ) = 2 ⎛ nπx ⎞ sin⎜ ⎟ a ⎝ a ⎠ h 2π 2 n 2 2mE n 2π 2 = 2 ⇒ E = En = 2 h a 2ma 2 energy for this system is quantized Particle in a box n=4 E n=3 ψ |ψn| 2 n n=2 n=1 (a) x= 0 (b) x= a x= 0 (c) Particle in an infinite potential well: a) Four lowest discrete energy levels; b) Corresponding wave functions c) Corresponding probability functions x= a En 2 n 2π 2 h = 2 ma 2 Reflection: Step potential ψ 1 (x ) = A1e jk x + B1e − jk x (x ≤ 0) 1 T.I.S.E ∂ 2ψ 1 ( x ) 2mE + 2 ψ 1 (x ) = 0 2 ∂x h k1 = 2mE h2 vi and vr are the magnitude of the incident and reflected wave velocity. 1 region II the Schrödinger equation ∂ 2ψ 2 ( x ) 2m + 2 (V0 − E )ψ 2 ( x ) = 0 2 ∂x h ψ 2 (x ) = A2e − k x + B2e k x (x ≥ 0) 2 k2 = 2m(V0 − E ) h2 ψ 2 (x ) = A2e − k x 2 B2=0 is zero due to fact that function must be finite everywhere and potential is a step function boundary x=0 2 ψ1(0)= ψ2(0) A1+B1=A2. jk1A1-jk1B1=-k2A2 ∂ψ 1 ∂x = x =0 ∂ψ 2 ∂x x =0 Continue ( )( B1 ⋅ B1* k 22 − k12 + 2 jk1k2 k 22 − k12 − 2 jk1k 2 = * 2 2 2 A1 ⋅ A1 k2 + k1 kinetic energy K.E.=mv2/2 ( k1 = ) 2mE = 2 h 2m h2 ( ( 1 2 mv ) 2 ) 2 2 1 ) v 2 mv = m 2 = h h 2 vr ⋅ B1 ⋅ B k − k + 4k12 k 22 R= = = 1.0 2 2 2 vi ⋅ A1 ⋅ A k 2 + k1 * 1 * 1 2 2 ( ) Barrier potential and tunneling quantum particles can tunnel trough the barrier and get out on the other side Tunneling E<U0 for leftside incident particle k0 = p√(2mE)/ħ, For wide barriers, The tunneling rate is a very sensitive function of L