* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Topic 7.1-Discrete energy and radioactivity
Background radiation wikipedia , lookup
Nuclear fusion wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Technetium-99m wikipedia , lookup
Ionizing radiation wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Valley of stability wikipedia , lookup
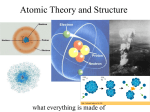
2/6/17 The Atom The earliest references to the concept of atoms date back to ancient India in the 6th century BCE. 7. Atomic and Nuclear Physics Chapter 7.1 The Atom The Atom A replica of Lavoisier's laboratory at the Deutsches Museum in Munich, Germany. The large lens in the center of the picture was used to focus sunlight in order to ignite samples during combustion studies. • In 1803, English instructor and natural philosopher John Dalton proposed that each element consists of atoms of a single, unique type, and that these atoms can join together to form chemical compounds. Robert Boyle (1627-1691) • Dalton used the concept of atoms to explain why elements always react in a ratio of small whole numbers - the law of multiple proportions - and why certain gases dissolve better in water than others. Antoine Lavoisier (1743-1794) The Atom John Dalton (1627-1691) The Atom-*KNOW THIS EXPERIMENT* • In 1897, he physicist J. J. Thomson, through his work on cathode rays, discovered the electron and its subatomic nature, which destroyed the concept of atoms as being indivisible units. • Thomson believed that the electrons were distributed throughout the atom, with their charge balanced by the presence of a uniform sea of positive charge (the plum pudding model). Although the term initially referred not only to matter but also to spiritual elements, it was later adopted in when modern Science started to develop. The Atom • In 1661, natural philosopher Robert Boyle suggested that matter was composed of various combinations of different "corpuscles" or atoms, rather than the classical elements of air, earth, fire and water. • In 1789 the term element was defined by the French nobleman and scientific researcher Antoine Lavoisier to mean basic substances that could not be further broken down by the methods of chemistry. In approximately 450 BCE, Democritus coined the term átomos (Greek: ἄτοµος), which means "uncuttable" or "the smallest indivisible particle of matter", i.e., something that cannot be divided further. J. J. Thomson • However, in 1909, Geiger and Marsden, two researchers under the direction of physicist Ernest Rutherford, bombarded a sheet of gold foil with helium ions and discovered that a small percentage were deflected through much larger angles than was predicted using Thomson's proposal. Ernest Rutherford (1856-1940) (1871-1937) Hans Geiger Ernest Marsden (1882-1945) (1889-1970) 1 2/6/17 The Atom-*KNOW THIS EXPERIMENT* • Rutherford interpreted the gold foil experiment as suggesting that the positive charge of an atom and most of its mass was concentrated in a nucleus at the centre of the atom (the Rutherford model), with the electrons orbiting it like planets around a sun. Rutherford s (or Geiger-Marsden) gold foil experiment à Expected results: alpha particles passing through the plum pudding model of the atom undisturbed. à Observed results: a small portion of the particles were deflected, indicating a small, concentrated positive charge. • Positively charged helium ions passing close to this dense nucleus would then be deflected away at much sharper angles The Atom • Most of the α-particles passed straight through the foil, but to Rutherford s surprise a few were scattered back towards the source. • Rutherford said that this was rather like firing a gun at tissue paper and finding that some bullets bounce back towards you! Consequences of the Rutherford s experiment • All of an atom's positive charge and most of its mass is concentrated in a tiny core. Rutherford called this the nucleus. • The electrons surround the nucleus, but they are at relatively large distances from it. • The atom is mainly empty space! Relative size of the nucleus and electric cloud Rutherford s model of the atom • Can we use Rutherford s model of the atom to explain the α-particle scattering? • The concentrated positive charge produces an electric field which is very strong close to the nucleus. • The closer the path of the α-particle to the nucleus, the greater the electrostatic repulsion and the greater the deflection. • Most α-particles are hardly deflected because they are far away from the nucleus and the field is too weak to repel them much. • The electrons do not deflect the α-particles because the effect of their negative charge is spread thinly throughout the atom. 2 2/6/17 Rutherford s model of the atom Rutherford s model of the atom • Using this model Rutherford calculated that the diameter of the gold nucleus could not be larger than 10-15 m. • Other experiments confirmed the existence of a nucleus inside the atom – a small, massive object carrying the positive charge of the atom. • The force that would keep the electrons in orbit was the electrical force between electrons and the positive nuclear charge – Coulomb s force. • According to the electromagnetic theory an accelerated charge would radiate electromagnetic waves and thus lose energy. • The electrons move in circular paths around the nucleus. But if they radiate and lose energy, then they would fall towards the nucleus. • because of this, Rutherford s model cannot explain way matter is stable, i.e., why atoms exist. The Bohr model Can you see any problems here? Bohr s model of the atom Bohr s postulates • The first attempt to solve the problem with Rutherford s model came from Niels Bohr, a Danish physicist, in 1911. • By examining the H atom, Bohr realized that the electron could exist in certain specific states of definite energy (energy levels) without radiating energy, if a certain condition was met by the orbit radius. • Bohr revised Rutherford's model by suggesting that the electrons were confined into clearly defined orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states. • The electron energy is thus discrete and not continuous. Niels Bohr (1885-1962) • An electron must absorb or emit specific amounts of energy to transition between these fixed orbits. Energy levels - evidence • Thomas Melvill was the first to study the light emitted by various gases. He used a flame as a heat source, and passed the light emitted through a prism. • An electron can only lose energy when it makes a transition from one state to another of lower energy. The emitted energy is then the difference of energy between the initial and final states. • The evidence for this is the absorption and emission spectra. Emission spectra Individual atoms, free of the strong interactions that are present in a solid, emit only certain specific wavelengths that are unique to those atoms. • Melvill discovered that the pattern produced by light from heated gases is very different from the continuous rainbow pattern produced when sunlight passes through a prism. • The new type of spectrum consisted of a series of bright lines separated by dark gaps. Emission spectrum of iron 3 2/6/17 Energy levels - evidence • This spectrum became known as a line spectrum. • Melvill also noted the line spectrum produced by a particular gas was always the same. Emission and absorption spectra n n • In other words, the spectrum was characteristic of the type of gas, a kind of "fingerprint" of the element or compound. n • This was a very important finding as it opened the door to further studies, and ultimately led scientists to a greater understanding of the atom. n n Emission and absorption spectra for the same gas Spectra can be categorized as either emission or absorption spectra. An emission spectrum is, as the name suggests, a spectrum of light emitted by an element. It appears as a series of bright lines, with dark gaps between the lines where no light is emitted. An absorption spectrum is just the opposite, consisting of a bright, continuous spectrum covering the full range of visible colors, with dark lines where the element literally absorbs light. The dark lines on an absorption spectrum will fall in exactly the same position as the bright lines on an emission spectrum for a given element, such as neon or sodium. Line spectra What causes line spectra? n n n n Line spectra n Planck and Einstein's quantum theory of light gives us the key to understanding the regular patterns in line spectra. n The photons in these line spectra have certain energy values only, so the electrons in those atoms can only have certain energy values. n This energy level diagram shows a very simple case. It is for an atom in which there are only two possible energy levels: You always get line spectra from atoms that have been excited in some way, either by heating or by an electrical discharge. In the atoms, the energy has been given to the electrons, which then release it as light. Line spectra are caused by changes in the energy of the electrons. Large, complicated atoms like neon give very complex line spectra, so physicists first investigated the line spectrum of the simplest possible atom, hydrogen, which has only one electron. Line spectra n The electron, shown by the blue dot, has the most potential energy when it is on the upper level, or excited state. n When the electron is on the lower level, or ground state, it has the least potential energy. n The diagram shows an electron in an excited atom dropping from the excited state to the ground state. n This energy jump, or transition, has to be done as one jump. It cannot be done in stages. n This transition is the smallest amount of energy that this atom can lose, and is called a quantum (plural = quanta). 4 2/6/17 Line spectra n n n The potential energy that the electron has lost is given out as a photon (particle of light). This energy jump corresponds to a specific frequency (or wavelength) giving a specific line in the line spectrum. This outlines the evidence for the existence of atomic energy levels. Calculating Energy n Energy = Planck’s Constant x frequency E = hf n h=6.63 x 10-34 Js Hydrogen atom energy levels Quantum physics provides the tools to compute the values of Rearranged… E1, E2, E3, etc…The results are: 5 4 n The databook also gives this equation solving for λ: c c à f = à E = h c à λ = h λ E λ c = fλ λ =h c E 3 2 1 En = -13.6 / n2 Energy Level Energy En (eV) 1 -13.6 2 -3.4 3 -1.51 4 -0.85 5 -0.54 These results DO DEPEND ON THE TYPE OF ATOM OR MOLECULE So, the difference in energy between the 3rd and 1st quantum state is: Ediff = E3 – E1 = -1.51 – (-13.6) = 12.09 (eV) When this 3à 1 atomic transition occurs, this energy is released in the form of electromagnetic energy. Example 4 In the preceding example, what is the frequency, wavelength of the emitted photon, and in what part of the EM spectrum is it in? E = 12.1 [eV]. First convert this to [J]. ⎛ 1.6x10-19 [J] ⎞ −18 12.1 [eV] ⎜ ⎟ = 1.94 x10 [J] ⎝ 1 [eV] ⎠ λ =h Nuclear Structure 8 c 3.00x10 = (6.63x10−34 ) = 1.0x10−7 m E 1.94x10−18 = 100nm This corresponds to UV rays! 5 2/6/17 Atomic structure Mass number and atomic number Electrons (negative particles) - e A – Mass number Protons (positive particles) - p Neutrons (uncharged particles) - n Z – Atomic number A Z X Element X Mass number = no. of protons + no. neutrons Particle Relative Mass Charge Location Proton 1 +1 Nucleus Neutron 1 0 Nucleus Electron 1/1800 -1 Electric cloud = no. of nucleons A=p+n Atomic number = no. of protons Z=p Atoms have no charge. So, no. electrons = no. protons e=p Elements n All materials are made from about 100 basic substances called elements. n An atom is the smallest piece of an element you can have. n Each element has a different number of protons in its atoms: ¨ it has a different atomic number (sometimes called the proton number). atomic number also tells you the number of electrons in the atom. ¨ The Isotopes Isotopes Isotopes are atoms that have the same number of protons but different number of neutrons. Hydrogen 1 1 H 1 proton 0 neutrons Deuterium 2 1 H 1 proton 1 neutron Hydrogen 1 1 Tritium 3 1 H n 1 proton 2 neutrons n H Deuterium 2 1 H Tritium 3 1 H Since the isotopes of an element have the same number, of electrons, they must have the same chemical properties. The atoms have different masses, however, and so their physical properties are different. 6 2/6/17 Evidence for neutrons Interactions in the nucleus n The existence of isotopes is evidence for the existence of neutrons because there is no other way to explain the mass difference of two isotopes of the same element. n By definition, two isotopes of the same element must have the same number of protons, which means the mass attributed to those protons must be the same. n Therefore, there must be some other particle that accounts for the difference in mass, and that particle is the neutron. n Electrons are held in orbit by the force of attraction between opposite charges. n Protons and neutrons (nucleons) are bound tightly together in the nucleus by a different kind of force, called the strong, short-range nuclear force. n It is this force that prevents the protons from repelling each other and breaking the nucleus apart. n There are also Coulomb interaction between protons due to the fact that they are charged particles. Radioactivity 7. Atomic and Nuclear Physics n In 1896, Henri Becquerel discovered, almost by accident, that uranium can blacken a photographic plate, even in the dark. n Uranium emits very energetic radiation - it is radioactive. Chapter 7.2 Radioactivity Henri Becquerel (1852-1908) In 1903, he shared the Nobel Prize in Physics with Pierre and Marie Curie "in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity". Radioactivity n Then Marie and Pierre Curie discovered more radioactive elements including polonium and radium. n Scientists soon realized that there were three different types of radiation. n Image of Becquerel's photographic plate which has been fogged by exposure to radiation from a uranium salt. Properties of Alpha, Beta and Gamma Radiation Marie Curie (1867-1934) These were called alpha (α), beta (β), and gamma (γ) rays from the first three letters of the Greek alphabet. Pierre Curie (1859-1906) 7 2/6/17 Properties of Alpha, Beta and Gamma Radiation The diagram shows how the different types are affected by a magnetic field. n n The alpha beam is a flow of positively (+) charged particles, so it is equivalent to an electric current. n The beta particles are much lighter than the alpha particles and have a negative (-) charge, so they are deflected more, and in the opposite direction. n n It is deflected in a direction given by the Right Hand Rule. (you know…from earlier) Ionizing Properties Being uncharged, the gamma rays are not deflected by the field. Alpha and beta particles are also affected by an electric field - in other words, there is a force on them if they pass between oppositely charged plates. Ionising Properties n α -particles, β -particles and γ -ray photons are all very energetic particles. n We often measure their energy in electron-volts (eV) rather than joules. (1 Joule = 6.24 x 1018 eV) n Typically the kinetic energy of an α -particle is about 6 million eV (6 MeV). n We know that radiation ionizes molecules by `knocking' electrons off them. n As it does so, energy is transferred from the radiation to the material. n The next diagrams show what happens to an α-particle Penetrating power of alpha radiation. n Properties of Alpha, Beta and Gamma Radiation Since the α-particle is a heavy, relatively slow-moving particle with a charge of +2e, it interacts strongly with matter. 105 n It produces about 1 x path in air. n After passing through just a few cm of air it has lost its energy. Penetrating power of beta radiation. n The β-particle is a much lighter particle than the α -particle and it travels much faster. n Since it spends just a short time in the vicinity of each air molecule and has a charge of only -le, it causes less intense ionization than the α -particle. n The β -particle produces about 1 x 103 ion pairs per cm in air, and so it travels about 1 m before it is absorbed. ion pairs per cm of its 8 2/6/17 Penetrating power of gamma radiation. n A γ-ray photon interacts weakly with matter because it is uncharged and therefore it is difficult to stop. n A γ -ray photon often loses all its energy in one event. n However, the chance of such an event is small and on average a γ -photon travels a long way before it is absorbed. Alpha, Beta and Gamma Radiation Detection of alpha radiation. Geiger-Muller (GM) tube n This can be used to detect alpha, beta, and gamma radiation. Geiger-Muller (GM) tube The `window' at the end is thin enough for alpha particles to pass through. If an alpha particle enters the tube, it ionizes the gas inside. This sets off a high-voltage spark across the gas and a pulse of current in the circuit. A beta particle or burst of gamma radiation has the same effect. n n n n Ionisation Chamber n n The ionization chamber is another detector which uses the ionizing power of radiation. The chamber contains fixed electrodes, which attract electrons and ions produced by the passage through the chamber of high-speed particles or rays. n When the electrodes detect ions or electrons, a circuit is activated and a pulse is sent to a recording device such as a light. Cloud and Bubble Chamber n n n n n n Have you looked at the sky and seen a cloud trail behind a high flying aircraft? Water vapor in the air condenses on the ionized exhaust gases from the engine to form droplets that reveal the path of the plane. A cloud chamber produces a similar effect using alcohol vapor. Radiation from a radioactive source ionises the cold air inside the chamber. Alcohol condenses on the ions of air to form a trail of tiny white droplets along the path of the radiation. The diagrams below show some typical tracks 9 2/6/17 Cloud and Bubble Chamber Stability n The α-radiation produces dense straight tracks showing intense ionization. n Notice that all the tracks are similar in length. n The high-energy β-ray tracks are thinner and less intense. n The tracks vary in length and most of the tracks are much longer than the α -particle tracks. n n The γ-rays do not produce continuous tracks. n n A bubble chamber also shows the tracks of ionizing radiation. The radiation leaves a trail of vapor bubbles in a liquid (often liquid hydrogen). n n If you plot the neutron number N against the proton number Z for all the known nuclides, you get the diagram shown here Can you see that the stable nuclides of the lighter elements have approximately equal numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. Heavier nuclei need more and more neutrons to be stable. Can we explain why? A plot of neutron number versus proton number is also called Segre plot. Stability n It is the strong nuclear force that holds the nucleons together, but this is a very short range force. n The repulsive electric force between the protons is a longer range force. n So in a large nucleus all the protons repel each other, but each nucleon attracts only its nearest neighbors. n More neutrons are needed to hold the nucleus together (although adding too many neutrons can also cause instability). n There is an upper limit to the size of a stable nucleus, because all the nuclides with Z > 83 are unstable. Radioactive decay equations Alpha decay Beta decay 4 2 He or 24α n An alpha-particle is a helium nucleus and is written n It consists of 2 protons and 2 neutrons. When an unstable nucleus decays by emitting an α -particle it loses 4 nucleons and so its nucleon number decreases by 4. Also, since it loses 2 protons, its proton number decreases by 2 The nuclear equation is n n n n A Z X → ZA−−42Y + 24α n n n Many radioactive nuclides decay by β-emission. This is the emission of an electron from the nucleus. But there are no electrons in the nucleus! n What happens is that one of the neutrons changes into a proton (which stays in the nucleus) and an electron (which is emitted as a β-particle). n This means that the proton number increases by 1, while the total nucleon number remains the same. n The nuclear equation is A Z Note that the top numbers balance on each side of the equation. So do the bottom numbers. A X→Z+1 Y+ −10e + ν Notice again, the top numbers balance, as do the bottom ones. € 10 2/6/17 The (anti)neutrino The (anti)Neutrino n While n The a nuclear equation balances, energy and momentum are not conserved, giving reason for another particle – the neutrino (or the antineutrino). n Basically, the momentum and energy escape in a tiny particle. n Neutrino = Italian - little neutral one (oh, cute…) antineutrino accompanies the electron, in beta decay. ( ν ) n The Neutrino accompanies the positron, in beta decay. (ν ) n In nuclear physics, the prefix “anti-” refers to an anti particle, that has some opposite € characteristic from it’s pair. € Beta decay The (anti)Neutrino n Neutrino has no electric charge n Was thought of to have no mass, but more recent experiments suggest there may be a minimal mass (smaller than an electron!) n Interacts weakly with matter, and is incredibly difficult to detect. n (I know…sounds made up, huh?) Decay chains n n n n A radio-nuclide often produces an unstable daughter nuclide. The daughter will also decay, and the process will continue until finally a stable nuclide is formed. This is called a decay chain or a decay series. Part of one decay chain is shown below A radio-nuclide above the stability line decays by β-emission. Because it loses a neutron and gains a proton, it moves diagonally towards the stability line, as shown on this graph. n n Gamma decay Gamma-emission does not change the structure of the nucleus, but it does make the nucleus more stable because it reduces the energy of the nucleus. n Decay chains n When determining the products of decay series, the same rules apply as in determining the products of alpha and beta, or artificial transmutation. n The only difference is several steps are involved instead of just one. 11 2/6/17 Half-life n n n n n n n n Suppose you have a sample of 100 identical nuclei. All the nuclei are equally likely to decay, but you can never predict which individual nucleus will be the next to decay. The decay process is completely random. Also, there is nothing you can do to `persuade' one nucleus to decay at a certain time. The decay process is spontaneous. Half-life n Iodine-131 is a radioactive isotope of iodine. n The chart illustrates the decay of a sample of iodine-131. n On average, 1 nucleus disintegrates every second for every 1000 000 nuclei present. Does this mean that we can never know the rate of decay? No, because for any particular radio-nuclide there is a certain probability that an individual nucleus will decay. This means that if we start with a large number of identical nuclei we can predict how many will decay in a certain time interval. Half-life n The half-life of a radioactive isotope is the time taken for half the nuclei present in any given sample to decay. To begin with, there are 40 million undecayed nuclei. 8 days later, half of these have disintegrated. With the number of undecayed nuclei now halved, the number of disintegrations over the next 8 days is also halved. It halves again over the next 8 days... and so on. Iodine-131 has a half-life of 8 days. Activity and Half-life n n n In a radioactive sample, the average number of disintegrations per second is called the activity. The SI unit of activity is the becquerel (Bq). An activity of, say, 100 Bq means that 100 nuclei are disintegrating per second. Activity and Half-life n So `half-life' has another meaning as well: The half-life of a radioactive isotope is the time taken for the activity of any given sample to fall to half its original value. n The graph shows how, on average, the activity of a sample of iodine-131 varies with time. n As the activity is always proportional to the number of undecayed nuclei, it too halves every 8 days. 12 2/6/17 Exponential Decay n n n Any quantity that reduces by the same fraction in the same period of time is called an exponential decay curve. The half life can be calculated from decay curves Take several values and then take an average 13