* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Untitled
Interactome wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biosynthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Proteolysis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Signal transduction wikipedia , lookup
Western blot wikipedia , lookup
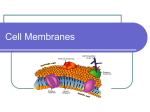
1 2 What defines all living systems is the ability to generate a chemical environment inside the cell that is different from the extracellular one. The plasma membrane separates the inside of the cell (cytoplasm) from the outside (or extracellular) environment. Membranes are also found surrounding many internal organelles, as we will see a bit later. What is the membrane made of, how does it assemble, and what are the forces that stabilize its structure? What are the chemical and physical properties of the membrane that influence its function? The membrane limits which molecules can get into and out of cells, but cells have to be able to exchange many different types of molecules between the inside and outside of the cell - large and small molecules, charged, polar and nonpolar ones. How do cells facilitate and control the exchange of molecules between the cytoplasm and environment? We will address these and other questions in the next lectures. 3 4 5 The major lipid components of cell membranes are phospholipids which comprise ~50% of animal cell membranes (by mass). Phospholipids are amphipathic molecules; this means that they have both hydrophobic and hydrophilic parts. Shown here is phosphatidylcholine (PC), the predominant phospholipid in cell membranes. It has a hydrophilic head group comprised of choline linked to glycerol via a phosphate group. The choline group of PC is positively charged at neutral pH, and the phosphate group is negatively charged. This makes PC electrostatically neutral. In contrast, some phospholipids have a net negative charge. Phospholipids also have a hydrophobic tail with 2 fatty acid chains (from 14-24 carbons). Often one chain is saturated (no double bonds) and the other is unsaturated (one or more cis double bonds). Each cis double bond creates a kink in the fatty acid tail. Other phospholipids include sphingomyelin and various aminophospholipids. These lipids differ in their head groups and the length of their fatty acid tails. As we will see, the chemical composition of the phospholipids (degree of saturation, type of head group, length of fatty acid chain) can influence the physical properties of the membrane. 6 The plasma membrane separates the inside of the cell (cytoplasm) from the outside (or extracellular) environment. The shapes of lipids and the way they pack with each other have a large effect on the structures that they form in water, or water-based solutions (referred to as aqueous, or water-containing). lipids will arrange themselves in water in the way that minimizes the amount of hydrophobic surface that is exposed. Since biological lipids are amphipathic, they arrange themselves so that the polar head group is in contact with water, but their hydrophobic tails are buried – this arrangement minimizes the exposure of hydrophobic surface. Cell membranes are made up mainly of a double layer of phospholipid molecules, also called a pair of leaflets. The hydrophobic tails are sandwiched by the hydrophilic heads forming a barrier to most water soluble molecules. Lipid bilayers form spontaneously as a result of the chemical properties of phospholipids and the presence of water. Note that this is not a polymer like DNA or protein, it’s an assembly withou covalent bonds based on the chemical properties of the phospholipid. 7 Much of the basis for the unique properties of membranes resides in the hydrophobic effect. As you learned from Dan, hydrophilic molecules (like acetone; top) dissolve readily in water because their polar groups make favorable interactions with the polar groups of water molecules. When hydrophobic molecules (like 2-methyl propane; bottom) are immersed in water, water molecules order themselves around the hydrophobic molecules, decreasing the entropy of the water molecules and therefore increasing the free energy of this process. Hydrophobic molecules will arrange themselves in a manner that minimizes the amount of hydrophobic surface in contact with water – this arrangement will minimize the number of water molecules that have to be ordered, minimizes the entropy loss, and therefore results in the least increase in free energy. Because acetone is polar, it can form favorable electrostatic interactions with water molecules, which are also polar. Thus, acetone readily dissolves in water. By contrast, 2-methyl propane is entirely hydrophobic. It cannot form favorable interactions with water and it would force adjacent water molecules to reorganize into icelike cage structures, which increases the free energy. This compound therefore is virtually insoluble in water. The symbol δ- indicates a partial negative charge, and δ+ indicates a partial positive charge. Polar atoms are shown in color and nonpolar groups are shown in gray. 8 A lipid bilayer has edges in which the hydrophobic lipid tails are exposed to water. Because the contact of hydrophobic molecules with water is energetically unfavorable, synthetic bilayers organize themselves to eliminate free edges. The free edges can be eliminated by forming a sealed compartment, which places the polar head groups in contact with water and buries the hydrophobic fatty acid tails inside the compartment and away from water. This spontaneous formation of sealed compartments is an amazing property that is fundamental to living cells; it arises because of the amphipathic properties of phospholipids, their shape, and the fact that these molecules exist in an aqueous environment. The hydrophobic effect is the major driving force for bilayer formation and the formation of sealed compartments - it is the configuration of lowest free energy which minimizes the amount of hydrophobic surface area in contact with water. 9 Here is a photograph taken with an electron microscope to visualize the tiny organelles within the cell. You can see the large nuclei – big circles at the bottom - and smaller organelles, all bounded by membranes. If you look closely you can even see ribosomes as small dots in the cytoplasm or attached to the ER. 10 The shapes of lipids and the way they pack with each other have a large effect on the structures that they form in water, or water-based solutions (referred to as aqueous, or water-containing). As we discussed on the previous slide, lipids will arrange themselves in water in the way that minimizes the amount of hydrophobic surface that is exposed. Since biological lipids are amphipathic, they arrange themselves so that the polar head group is in contact with water, but their hydrophobic tails are buried – this arrangement minimizes the exposure of hydrophobic surface. Lipids with two fatty acid tails (like those found in most biological membranes), have a cylindrical shape; the packing arrangement that minimizes contact between their fatty acid tails and water is a bilayer where the hydrophobic tails are in the middle of the sandwich and the polar head groups are on the surface interacting with water. Lipid molecules with one fatty acid chain and a large polar head group have an overall shape that is conical, so when these types of lipids pack together they form a micelle in which the tails are inside and the polar head groups are in contact with water. Most detergents (like the dish and laundry detergents we use) have a charged head group and a long hydrocarbon chain, so they form micelles in aqueous solution. When detergents are added to a solution containing non-polar molecules, the non-polar molecules partition into the interior of the micelle, where they are shielded from water and can interact with the hydrocarbon tails of the detergent. 11 12 Although we draw them as static structures, biological membranes are very dynamic because individual phospholids in bilayers are highly mobile. The two halves of the bilayer are referred to as leaflets. Individual phospholipids can rotate about their long axis, their fatty acid tails can flex, and they can diffuse laterally in the plane of the bilayer. Lateral diffusion is so fast that a phospholipid will move through the membrane from from one end of a bacterial cell to the other (~2 mm) in 1 second. By contrast, exchange of phospholipids between the two leaflets - called flip-flop - is rare; on average it occurs less than once per month for a single phospholipid. Think of the membrane lipids as constituting a two-dimensional solvent for the proteins in the membrane, just as water constitutes a threedimensional solvent for proteins in an aqueous (i.e. liquid) solution. 13 14 The fluidity of a lipid bilayer depends on both its composition and its temperature. An example of this difference in fluidity and melting temperature can be seen with two fatty acids: oleic acid and stearic acid. These two fatty acids have the same number of carbons (18), but they differ dramatically in their fluidity and melting temperature. At room temperature oleic acid is fluid-like (olive oil) and stearic acid is solid-like (candle wax); the melting temperature of oleic acid is lower than that of stearic acid because oleic acid is unsaturated and cannot pack in as orderly a manner as saturated stearic acid. 15 16 Lipids with shorter chains are less stiff and less viscous because they have less surface area to undergo stabilizing van der Waals interactions with neighboring hydrophobic chains. The degree of saturation of the fatty acid chains also affects the melting temperature and membrane fluidity. Fatty acids that are saturated, containing no double bonds, are straight and can pack more tightly than those that have double bonds. The kinks in the unsaturated chains simply make it more difficult to pack them in an orderly manner. As a consequence, the melting temperature of bilayers containing lipids with saturated fatty acids is higher than the melting temperature of bilayers containing lipids with unsaturated fatty acids. Additionally, the fluidity of bilayers containing lipids rich in unsaturated fatty acids is greater than the fluidity of bilayers containing lipids rich in saturated fatty acids. 17 18 The picture on the left shows photographer Miles Morgan on fire. 19 Many biological processes (e.g. membrane transport and some enzyme activities) are influenced by how readily lipids move in the plane of the bilayer – referred to as membrane fluidity. Membranes in which the lipids are more mobile are more fluid. Both chemical composition and temperature have an influence on fluidity. As the temperature is raised, a synthetic bilayer made of one type of phospholipid undergoes a phase transition (an abrupt change in one or more physical properties; like the one Dan told you about that occurs when ice melts to form water) from a solid-like state to a more fluid-like state at a characteristic melting point temperature (abbreviated as Tm). At low temperatures the lipids within the bilayer are well-ordered, packed into a crystal-like arrangement in which the lipids are not very mobile and there are many stabilizing interactions. As the temperature is raised, these interactions are weakened, and the lipids are in a less ordered, liquid-like state. A sharp phase transition is a problem for the membrane. 20 What we don’t want to happen: our membranes solidify in the cold, leaving us solid like this Jabba the Hutt made of butter. 21 Instead, what we need to do is flatten the curve so the membrane does not undergo a sharp phase transitions. How do cells do this?? 22 The answer is cholesterol! Cholesterol is a major component of eukaryotic cells. The ratio of cholesterol to lipid molecules in a the plasma membrane of eukaryotic cells can be as high as 1:1. Cholesterol is a member of a class of natural products called steroids, characterized by a 4 ring structure. Although all steroids are based on the same scaffold, they can affect different biological processes, ranging from the development of secondary sex characteristics (testosterone and estrogen) to inflammation (corticosteroids). Like phospholipids, cholesterol is an amphipathic molecule; it has a polar head that contains a hydroxyl group. The rest of cholesterol is hydrophobic, consisting of a rigid 4 ring steroid structure and a hydrocarbon tail. The 4 ring structure makes most of the cholesterol molecule very rigid because bonds between atoms in a ring are not free to rotate as a result of the geometric constraints of being in a ring. 23 Cholesterol inserts into the bilayer with its polar head group adjacent to the head group of the phospholipids (an arrangement that allows favorable interactions between polar groups) and with its nonpolar component interacting with the fatty acids tails of the lipids (via favorable van der Waals contacts). Cholesterol acts as a bidirectional regulator of membrane fluidity because at high temperatures, it stabilizes the membrane and raises its melting point, whereas at low temperatures it intercalates between the phospholipids and prevents them from clustering together and stiffening. 24 High Temperatures. Cholesterol has a substantial influence on membrane fluidity. At high temperature, the membranes are prone to movement. The rings of cholesterol is quite rigid. The rings of the cholesterol molecule interact with the fatty acid chains near the lipid head group, restricting the mobility of these chains, which decreases the mobility of the lipids and reduces membrane fluidity. The rigid, flat steroid rings interact with—and partly immobilize—those regions of the hydrocarbon chains closest to the polar head groups. By decreasing the mobility of the first few CH2 groups of the hydrocarbon chains of the phospholipid molecules, cholesterol makes the lipid bilayer less deformable in this region and thereby decreases the permeability of the bilayer to small water-soluble molecules. 25 High Temperatures Cholesterol interacts differentially with different membrane lipids, associating particularly strongly with saturated, high-melting phosphoand sphingolipids and particularly weakly with highly unsaturated lipid species. An example of cholesterol binding to sphingolipids such as sphingomyelin shown here. They hydroxyl on cholesterol forms a hydrogen bond with the amino group of the head of sphingomyelin, which orients cholesterol with respect to sphingomyelin. This complex helps with rigidity in the membrane by generating a larger rigid structure. Note that cholesterol is an asymmetric molecule displaying one face that interacts with sphingolipid, and a second face that can interact with proteins in the membrane. 26 Low Temperatures At the high concentrations found in most eukaryotic plasma membranes, cholesterol also prevents the hydrocarbon chains from coming together and crystallizing. In this way cholesterol inhibits possible phase transitions. At low temperatures: membrane packing with many van der waals interactions makes a solid between the tails. Cholesterol, with its kinked tail, disrupts the packing. There are fewer van der Waals interactions, and the membrane is more fluid. The influence of cholesterol on membrane properties is critical for the normal functioning of eukaryotic cells. 27 Eat your cholesterol! 28 Now let’s think about proteins. Many proteins (purple) are directed to the plasma membrane of the cell, where they play important functions. As we will see in the next lecture, some of the proteins in the plasma membrane play critical roles in transporting molecules across the membrane, whereas others are involved in communicating information from the outside of the cell to the inside. To give you a feel for the scale of membranes, proteins, and bonds: (1) The lipid bilayer is ~ 5 nm think. (2) A typical membrane protein is ~ 10 nm long. (3) A covalent bond is ~ 0.1 nm. (4) A cell is 2-4um (0.002mm; C. elegans, E. coli, yeast) to 6 inches (ostrich egg); an amoeba is about 500um. Skin cell is about 30um. Neurons vary in size from 4 microns (.004 mm) to 100 microns (.1 mm) in diameter. But their length varies from a fraction of an inch to several feet (5) Cell: 2um – 500um 29 29 30 31 For proteins to span the membrane, they need to overcome a problem: the charge of the protein backbone. Remember that the backbone of amino acids has a dipole that can interact with water but does not interact favorably with the hydrophobic lipids of the membrane. Because of the hydrophobic effect, the lipids in the membrane do not interact favorably with the dipole of the peptide backbone. 32 The solution! Transmembrane proteins commonly use alpha-helices to span the bilayer. These transmembrane helices are rich in hydrophobic amino acids so that the side chains that protrude from the helix can make favorable van der Waals interactions with the fatty acid tails of the lipids. These side chains also shield the backbone from the lipid molecules. Since there is no water in the middle of the bilayer, the hydrogen bonds that stabilize the helical structure are strong because they have no competition for hydrogen bonding with water (as they would in aqueous solution). Thus, the helical structure is favored in the hydrophobic environment of the bilayer. Approximately 20 amino acids are required to span the bilayer in a helical structure. Scientists can take advantage of knowledge of helical propensity of certain amino acids (the preference they have for or against being in an alpha-helical structure) and hydrophobicity to predict transmembrane domains in proteins. 33 Here we can see the alpha helix from the top and from the side. The side chains, in light purple, surround and shield the backbone, shown in dark purple. 34 Here are two transmembrane structures. The one of the left is a beta barrel composed of beta sheets. The one on the right is composed of alpha helices. Both can be placed in the membrane in an orientation that shields the peptide backbone as well as the termini. In other words, 1 and 3 both work, but 2 and 4 leave the backbone (at the unstructured loops and at the ends of the alpha helices) and probably the termini exposed to the hydrophobic environment of the membrane. The unstructured loops (the segments that aren’t part of either alpha helices or beta sheets) leave the peptide backbone exposed (its hydrogen bonds aren’t satisfied by hydrogen bonds to other peptide backbone atoms), and as a result, it is unfavorable to bury these unstructured loops in the hydrophobic membrane. 35 Although most transmembrane proteins are alpha helices, a beta barrel can also work. The R groups that emanate from the sheet solve the hydrophobicity problem analogously to the alpha helix. In class we also discussed a protein that was lying on the surface of the membrane and not spanning the membrane. Do you remember what sort of configuration a cell uses to generate that orientation? 36 This answer assumes that the back side of each structure is consistent with the view that is shown above (i.e., it assumes that the reverse side of GFP is polar and the reverse side of FhuA also contains a non-polar band). GFP would not likely be embedded in a membrane because it contains a number of surface charges. Each of these surface charges would need to break hydrogen bonds to water in order to become buried in the hydrophobic environment of a membrane, which is enthalpically unfavorable. FhuA, conversely, has a large band of uncharged (i.e., nonpolar) surface that could become buried in the hydrophobic environment of a membrane due to the hydrophobic effect. 37 38 39 40 We talked about individual proteins and individual lipids. Now let’s think about the whole cell. Are proteins randomly distributed in the membrane? Or is there organization? And if there is organization, how does the cell do it?? 41 42 43 Shown here is a microdomain with a different cohort of lipids than the surrounding area, and different proteins. The microdomain associates together because of the chemical properties of the lipids. For example, longer lipids tend to associate with each other. Proteins with longer alpha helices are more likely to segregate into portions of the membrane with longer lipids. Because of interactions between lipids and cholesterol or proteins and cholesterol, cholesterol is enriched in some regions of the membrane over others. This kind of partioning can promoter protein – protein interactions by raising the concentration of specific factors. These microdomains are quite small and probably most of them exist only transiently. A lipid raft is a controversial lipid domain that you may hear about. It is thought to be a large and stable structure. People have seen lipid rafts in the lab, but it’s a mystery whether they really exist in cells! Fun Fact: This is an actual protein structure: the human type 3 somatostatin receptor, which possesses two cholesterol binding domains. 44 The analysis of proteins in living cells was revolutionized by GFP: green fluorescent protein. GFP is derived from a jelly fish A. victoria. It can be cloned like any other piece of DNA and, what is relevant here, its sequences can be fused to other proteins to generate a hybrid protein. By visualizing GFP in living cells, we can ‘watch’ the other protein, in this case, the brown protein. You’ll hear how GFP works if you take LS1b. 45 Let’s return to the question we were addressing. By tagging two different proteins with two fluorescent proteins, we can track if they localize together or apart. In this experiment two proteins were tagged and you can see if they behave similarly or differently. How dynamic are the two domains with the red vs green proteins? Note that this is a movie of a real cell – this is not a cartoon. It’s the actual data from an article. The authors have labelled two proteins with green fluorescent protein and a red fluorescent protein. 46 47