* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Packet 2- Chemistry of Life
Fluorescence correlation spectroscopy wikipedia , lookup
Crystallization wikipedia , lookup
Drug discovery wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Atomic theory wikipedia , lookup
Water pollution wikipedia , lookup
Analytical chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Stoichiometry wikipedia , lookup
Electrochemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Reverse osmosis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transition state theory wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Signal transduction wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Protein adsorption wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Western blot wikipedia , lookup
Chemical biology wikipedia , lookup
Physical organic chemistry wikipedia , lookup
History of molecular biology wikipedia , lookup
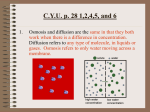
Lecture 2: Chemistry of Life Reading: Silverthorn (6th ed) Chapters 2, 4 Why study chemistry? You are a walking chemical reaction. To understand HOW your body works, you must have a general understanding of basic chemistry. This lecture will provide you with that background. Molecular structure determines function 1. Proteins are molecular machines and the way they are SHAPED determines what they can DO. A. Review: Protein structure i. Primary structure: String of amino acids ii. Secondary structure: The string is folded in some way (beta pleated sheets or alpha helices) iii. Tertiary structure: The folded shape FOLDS on itself iv. Quaternary structure: More than one protein with tertiary structure COMBINES B. Exposure to acids or bases (or heat) can affect the ability of each amino acid to form chemical bonds with others. C. Even exposure to different concentrations of ions can affect the shape of a protein. D. Binding with other substances can also affect the shape of a protein...(example: transporters!) 2. Enzymes are proteins that catalyze chemical reactions 3. Receptors are proteins that bind to signal molecules (like hormones) and then initiate some sort of cellular response. 4. Transporters are proteins that help substances move in and out of a cell. Chemical reactions proceed to equilibrium 1. Chemical reactions can go either direction, and they continue until it reaches equilibrium. A. Equilibrium happens when the rate at which a forward reaction happens is the same as the rate at which the backward reaction happens. H2O + CO2 ↔ H2CO3 ↔ H+ + HCO3 B. Law of mass action says that the ratio of products to reactants in a reaction at equalibrium is always the same. C. Significance? If you remove (or add) product or reactant, you can MOVE the direction that the equation will happen. 2. Acids and bases usually exist in equilibrium. A. (You might want to REVIEW your understanding of pH. Check out our website, which directs you to the Bio 1 lecture on Chemistry if you want a refresher.) 3. Buffers are substances that manage to prevent drastic changes in pH...they do this by removing (or adding ) H+ ions from/to a solution. Concentration gradients provide energy to “make stuff happen” 1. Concentration is defined as the number of solute molecules per unit volume of the solution. 2. Units of concentration are particles/volume A. In chemistry, concentration is measured in molarity (moles/liter) calculate the molarity of each of these solutions: 2 moles NaCl in 1 L of H2O 3 moles C6H12O6 in 1 L of H2O B. In physiology, concentration is measured in osmolarity (particles/mole/L). This important distinction is made because many substances, like NaCl, dissociate (split apart) in water. calculate the osmolarity of each of these solutions: 2 moles NaCl in 1 L of H2O Bio 7: Human Physiology 3 moles C6H12O6 in 1 L of H2O 5 Spring 2014: Riggs Diffusion 1. Random molecular movement from areas of high concentration to areas of lower concentration. 2. Diffusion is the movement of molecules DOWN a concentration gradients. (http://highered.mcgraw-hill.com/sites/0072495855/ student_view0/chapter2/animation__how_diffusion_works.html) 3. Enables the maintenance of the “relatively stable internal environment”! ↓ conc water ↑ conc water ↑ conc solute ↓ conc solute Osmosis 1. Osmosis is the movement of water molecules across a semi-permeable membrane... (http://highered.mcgraw-hill.com/ sites/0072495855/student_view0/chapter2/animation__how_osmosis_works.html) 2. Movement of water can create OSMOTIC PRESSURE! A. Conclusion: Osmolarity describes the solution, but not how WATER will affect the cell…what the water does depends on: i. Osmolarity of both “sides” ii. Qualities of the MEMBRANE separating the sides. iii. What particles can pass through, and what particles cannot?! a. # Penetrating solutes on each side (can pass through the membrane) b. # Non-penetrating solutes on each side(cannot pass through the membrane) B. Tonicity describes how WATER will affect a cell… i. In a hypotonic solution, the water rushes into the cell, and it swells. ii. In a hypertonic solution, the water rushes out of the cell, and it shrinks (crenates) iii. In an isotonic solution, the water does not move…the cell stays the same size. Bio 7: Human Physiology 6 Spring 2014: Riggs External Brain 2: Chemistry of Life Study Guide Questions 1. Be able to do questions similar to the Osmosis worksheet (posted under “Supplemental Resources” on 8/30 in the Service Station.) 2. Compare and contrast osmolarity and molarity. 3. Compare and contrast osmolarity and tonicity. 4. Describe the various fluid compartments in the body. Be able to address the relative solute concentrations of K+, Na+, Ca2+, Cl-, and proteins in each fluid compartment. 5. Given a solution’s molarity, be able to determine its osmolarity, and vice versa. 6. Be able to discuss the concept of “osmotic pressure”. 7. Be able to interpret a graph from the dialysis bag experiment. 8. What is concentration? 9. Distinguish between osmolarity and molarity. 10.Given information about whether a substance dissociates in water or not, be able to calculate its molarity AND its osmolarity. 11. What is a mole? 12.Define diffusion. 13.Distinguish between diffusion and osmosis. 14.Illustrate the different levels of protein structure. 15.Link protein structure to its function. 16.Describe different conditions that might change the structure of a protein. What might the consequences of this be? 17.What is pH? 18.Compare and contrast the following cellular structures: enzymes, receptors, transporters 19.Define chemical equilibrium. 20. Given a chemical equation, determine the direction the reaction will proceed, given different scenarios. 21.What is the law of mass action? 22.What is a buffer? Why are buffers important? Bio 7: Human Physiology 7 Spring 2014: Riggs