* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A role for the Drosophila Bag-of-marbles protein in
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
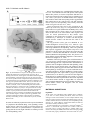
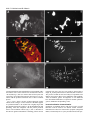
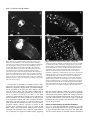
Development 121, 2937-2947 (1995) Printed in Great Britain © The Company of Biologists Limited 1995 2937 A role for the Drosophila Bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells Dennis McKearin* and Benjamin Ohlstein Department of Biochemistry, University of Texas-Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235-9038, USA *Author for correspondence (e-mail: [email protected]) SUMMARY Cell differentiation commonly dictates a change in the cell cycle of mitotic daughters. Previous investigations have suggested that the Drosophila bag of marbles (bam) gene is required for the differentiation of germline stem cell daughters (cystoblasts) from the mother stem cells, perhaps by altering the cell cycle. In this paper, we report the preparation of antibodies to the Bam protein and the use of those reagents to investigate how Bam is required for germ cell development. We find that Bam exists as both a fusome component and as cytoplasmic protein and that cytoplasmic and fusome Bam might have separable activities. We also show that bam mutant germ cells are blocked in differentiation and are trapped as mitotically active cells like stem cells. A model for how Bam might regulate cystocyte differentiation is presented. INTRODUCTION germline stem cell divides to produce two daughters (Fig. 1A). One daughter remains a stem cell while the other becomes a cystoblast. The cystoblast divides precisely four times with incomplete cytokinesis at each mitosis to produce a cluster of sixteen interconnected sister cells called cystocytes. Cystocytes remain connected to one another by stable intercellular bridges, termed ring canals (King, 1970; Robinson et al., 1994), that form at the site of contact between the mitotic cleavage furrow and the spindle equator (King, 1970; Telfer, 1975). One cystocyte becomes the oocyte while its fifteen sisters become nurse cells that supply the developing oocyte with nutrients and biomolecules for oocyte maturation and subsequent embryonic development. Each round of cystocyte mitosis is accompanied by the growth of a germ cell-specific organelle called the fusome (King, 1970; Telfer, 1975; Storto and King, 1989; Lin et al., 1994). It is found as a a spherical dot in the cystoblast and grows to become an elongated, branched structure in the cystocyte cluster (i.e. the cyst). Stem cells also contain a similar organelle which Lin et al. (1994) have designated the spectrosome. Fig. 1B shows a drawing of a fusome connecting a cluster of eight cells in the germarium of a wasp (Maziarski, 1913); Drosophila fusomes are essentially indistinguishable from wasp fusomes. The fusome acts as a pole of each mitotic spindle by capturing a centriole and, in this capacity, serves to orient the planes of cell division at each cystocyte mitosis. Since the syncytium produced by four rounds of cystocyte divisions represents a clear example of asymmetric differentiation among the mitotic sisters (i.e. oocyte versus nurse cell differentiation), it has been hypothesized that cystocyte Stem cell divisions are inherently asymmetric since they produce mitotic daughters from which specialized cell types will differentiate (Horvitz and Herskowitz, 1992; Hall and Watt, 1989). Mutations in genes required for asymmetric segregation of differentiation factors or in genes that are targets of such factors could block cellular differentiation and produce mitotic daughters that would inappropriately continue to divide like stem cells. Examples of these kinds of mutations might account for some types of leukemias and other neoplasms that are composed of blast-like cells (Lynch, 1995; Gondos, 1987a,b; Hall and Watt, 1989). Genetic screens for mutations that cause the accumulation of specific cell types have proven useful for identifying genes required for cell differentiation (Horvitz and Herskowitz, 1992; Hartwell and Weinert, 1989). In many cases, careful analysis of the points of arrest have provided insight into when the affected gene products begin to act and what biochemical functions they might influence to regulate steps of a differentiation pathway. The Drosophila germ cell lineage The germ cell lineage of Drosophila (Spradling 1993a,b) is an attractive model to identify the mechanisms of asymmetric cytoplasmic distribution during cell division since it affords genetic and molecular approaches. For example, mutations in many genes required for germline development are viable but sterile and mutations that produce apparent arrest of germ cell development have been identified. The process of oogenesis in the adult fly begins when a Key words: Drosophila, bag of marbles, cytoblast, stem cell, cell cycle, mitosis, germ cell, fusome 2938 D. McKearin and B. Ohlstein Fig. 1. (A) Schematized view of germ cell lineage from stem cells through the formation of 16 interconnected cystocytes. (B) A drawing of the fusome, which appears as the dark branched organelle at the boundaries of interconnected cystocytes of wasp germ cells. Five mitotic spindles and the complements metaphase chromosomes are also illustrated. The original drawing appears in Mazurski, 1915; this copy was reproduced from Telfer, 1975. (C) A schematic view of a wild-type germarium from Drosophila melanogaster. The numbers 1, 2A, 2B, 3 refer to Germarial Regions as described in King (1970). Region 1 contains stem cells, cystoblasts and 2-cell, 4cell and 8-cell clusters of cystocytes. In region 2A lie the recently completed 16-cell clusters while region 2B contains lens-shaped clusters of cystocytes that stretch across the full width of the germarium as they are becoming surrounded by migrating follicle cells. Germarial region 3 contains a spherical egg chamber with a complete complement of follicle cells arranged in an epithelial monolayer surrounding 15 nurse cells and an oocyte. TF, terminal filament cells. divisions are inherently asymmetric with respect to distribution of cytoplasmic determinants (King, 1970; Spradling, 1993b). In this context, it has been exciting to learn that fusome distribution at the time of cystoblast mitosis is asymmetric suggesting that the fusome might contribute to marking cell fate (Lin and Spradling, 1995). Recent investigations have established that Spectrin (Spc; Lee et al., 1993) and Hu-li tai shao (Hts) protein, a Drosophila adducin-like protein, are fusome components and that hts is required for normal fusome assembly and cyst formation (Lin et al., 1994). Cystocytes in hts mutant females fail to complete four rounds of mitosis and consequently produce egg chambers that contain between two and ten nurse cells and frequently lack an oocyte (Yue and Spradling, 1992). Finally, no fusome forms in hts germ cells (Lin et al., 1994). Germ cell divisions take place in the germarium at the anterior tip of the ovary (Fig. 1C). The germarium has been divided into four regions (Mahowald and Kambysellis, 1980) that correspond to the various stages of cyst development. Stem cell divisions occur at the anterior end in region 1 and cysts are forced posteriorward as they mature. Upon completion of the fourth round of mitosis, a cyst becomes surrounded by a monolayer of somatic cells that migrate inward from the somatic cells that line the walls of the germarium. The bag-of-marbles (bam) gene was identified in a Pelement mutagenesis screen (Cooley et al., 1988) as a gene required for progression through the early steps of the germ cell lineage (McKearin and Spradling, 1990). The predicted Bam sequence suggested a novel protein except for a weak similarity to the Drosophila Otu protein and a motif near the protein’s C terminus that matched the consensus for PEST domains (McKearin and Spradling, 1990). The presence of a PEST domain suggested that Bam might have a short intracellular half-life (Rogers et al., 1986). Mutations in the bam gene arrest germ cell differentiation at very early stages of both oogenesis and spermatogenesis in a manner that suggests bam is required for the differentiation of germline stem cell daughters (McKearin and Spradling, 1990). In the absence of bam function, these cells continue to divide like stem cells producing germ cell tumors that characterize the mutant phenotype. In this paper, we report results that corroborate these hypotheses. We show that Bam is a fusome protein. We find that germ cells require Bam to progress beyond the cystoblast and, in its absence, germ cells become trapped as mitotically active non-differentiating cells. Finally, we present evidence that the initial requirement for Bam is not dependent on an intact fusome. Instead we suggest that it is associated with cytoplasmic Bam that begins to accumulate when the cystoblast initiates mitosis and disappears after the fourth cystocyte division. MATERIALS AND METHODS Fly stocks All fly stocks were maintained under standard culture conditions. y;ry506 and w1118 stocks (Lindsley and Zimm, 1992) were used as wild-type controls. bam86 mutation is a near complete deletion of the bam gene coding sequence and will be described elsewhere. The hts1 and hts2 mutations have been described by Yue and Spradling (1992) and Lin et al. (1994). Balancer chromosomes SM6b and TM3 are described in Lindsley and Zimm (1992). hts;bam mutant flies were constructed by crossing hts1/SM6b;ry506 males to +/+;bam86/TM3 virgin females. hts1/+;bam86/+ males were recovered in the F1 progeny and mated to +/SM6b;+/TM3 virgin females. Sib-mating hts1/SM6b;bam86/TM3 males and virgin females recovered from progeny established a stock from which hts1;bam86 Role of Drosophila Bag of marbles protein 2939 females could be isolated for ovary examinations. The same mating strategy was used to establish stocks of hts2/SM6b; bam86/TM3. Preparation of antibodies Bacterially expressed protein was produced from a pET vector (Novagen) containing a fragment of the bam gene. The recombinant protein carried a 10-residue poly(His) tag that was used for Ni2+column affinity purification of the fusion protein according to manufacturers instructions (Novagen). The PmlI-SacII fragment from a near full-length bam cDNA clone was ligated as a blunt-ended fragment into BamHI site of the pET16b polylinker; BamHI ends were made blunt by Klenow fill-in prior to ligation. The resulting clone encodes an open reading frame that includes 23 vector-encoded amino acids and residues 5-140 of the predicted Bam sequence (McKearin and Spradling, 1990). A second His-tagged recombinant protein was constructed by ligating the PmlI-HindIII cDNA fragment into pET16b. This fusion protein contains codons 5-405 of the predicted Bam polypeptide. Affinity-purified recombinant protein was injected into mice in the amount of 75 µg protein/injection. Antisera were tested for immunoreactivity on immunoblots against fusion protein, fulllength in vitro translated Bam and ovarian extracts from wild-type and bam86 flies. Antisera recovered from mice injected with either recombinant Bam antigen produced similar immunoreactivities. A total of seven mice produced Bam-positive antisera (15 total immunized). Immunoblots were performed as described previously (Christerson and McKearin, 1994); antisera were used at 1:7500 dilution. Secondary antibodies conjugated to alkaline phosphatase and the chemiluminescent substrate CSPD were used according to manufacturers instructions (Tropix) to detect membrane-bound antigens. Immunohistochemistry Ovaries from w1118 or bam mutant females were dissected into Drosophila Ringer’s and the tips of the ovaries were teased open to enhance antibody penetration of the tissue. Ovaries were fixed for 2030 minutes at 24°C in 0.3 ml of 1× PBTF/0.9 ml heptane. 1× PBTF is 3 mM NaH2PO4, 7 mM Na2HPO4, 130 mM NaCl, 0.125% TWEEN 20 and 4% formaldehyde (methanol-free; Pella Scientific). Ovaries were then rinsed 3× in PBT and washed 3× for 10 minutes/wash in PBT. The tissues were incubated for 60 minutes in PBTA (PBT+1.5% BSA). This solution was replaced with fresh PBTA containing the desired antibodies at an appropriate dilution (anti-Bam antisera were used at 1:2000) and incubated overnight at 4°C with gentle agitation. To test antisera specificity by soluble antigen competition, antisera (1:1000) were incubated with 0.5 µg of affinity-purified His-tagged Bam or 0.5 µg of His-tagged fusion protein for another Drosophila ovarian protein for 30 minutes at 24°C before addition of fixed ovaries. The reactions were performed as described above from this point on. We have noted that detection of Bam antigen, especially fusome associated Bam, is sensitive to fixation conditions and perhaps other factors since a fraction of germaria in a given sample are not stained by Bam antisera. BrdU incorporations were carried out essentially as described in Gönczy (1995). Briefly ovaries or testes were dissected from animals into 1× Drosophila Ringer’s solution. When sufficient organs had been collected, the buffer was removed and replaced with fresh Drosophila Ringer’s/20 µM BrdU and incubated for the appropriate time in the dark. At the end of the period of incorporation, the Ringer’s/BrdU was removed, organs were washed three times for 10 minutes in fresh Drosophila Ringer’s and fixed in 1 volume of 1× Drosophila Ringer’s/4% formaldehyde and 3 volumes of heptane with shaking for 20 minutes. After washing the fixation solution from the organs, they were incubated for 30 minutes in 2 N HCl. HCl was removed and the tissues were neutralized by incubating for 2 minutes in 100 mM Na2B2O4. The organs were then washed three times for 10 minutes each in 1× PBS/0.1% Triton X-100. Incorporated BrdU was detected by incubating the processed organs with anti-BrdU according to manufacturer’s suggestions and detecting with antimouse secondary antibodies conjugated to the fluorophore Cy3 (Jackson Immunoresearch Inc.). Microscopy Microscopy was performed on a Zeiss Axiophot microscope equipped with phase and differential interference contrast objectives. Confocal images were collected using the MRC 600 system (Bio-Rad Microsciences Division) attached to a Zeiss Axioplan microscope. Confocal images were photographed using a Polaroid FreezeFrame Video Recorder. The images recording ovaries double-labeled with antiSpectrin and anti-Bam shown in Fig. 4C were collected on a BioRad MRC1000 confocal microscope. To examine the cytology of ovaries, tissue was dissected in 1× PBS, and fixed with 4% paraformaldehyde in 1× PBS; ovarioles were teased apart to expose the egg chambers. Electron microscopy was performed on a JEOL 120 kV electron microscope at the UT-Southwestern Center for Microscopy. RESULTS Antibodies against Bam Previous investigations suggested that Bam was required for progression of germ cells beyond the cystoblast/2-cell cystocyte stage (McKearin and Spradling, 1990). As a first step to identify specific aspects of early germ cell differentiation that are dependent on Bam activity, we prepared antibodies against Bam. Polyclonal antisera were collected from mice immunized with recombinant proteins that contained segments of the predicted Bam sequence (see Material and Methods). Bam-positive antisera reacted with a single band of ~Mr 55,000 (Fig. 2, lane 3) in extracts derived from coupled in vitro tran- Fig. 2. Immunoblot of anti-Bam.m2 antiserum (1:7500) reacted against lanes containing protein from bam86 ovaries (lane 1), wildtype ovaries (lane 2), in vitro translation (IVT) extract programmed with bam mRNA (lane 3), IVT extract programmed with mRNA for an irrelevant ovarian protein (lane 4) and yeast cells expressing a LexA-Bam fusion protein (lane 5). The very slight difference in migration between the bands in lanes 2 and 3 probably does not represent authentic molecular mass differences since the shift is not reproducible on other immunoblots of similar samples. The faint band visible in lane 3 at ~ 30,000 Mr represents reaction of the mouse polyclonal serum against a contaminating protein; a band of the same Mr and that comigrated with lysozyme was detected in molecular mass marker lanes (not shown). The approximate positions of molecular mass marker proteins is indicated on the right side of the figure. 2940 D. McKearin and B. Ohlstein µ µ µ µ scription-translation extracts that had been programmed with a bam cDNA clone. These antisera also reacted with a band of ~Mr 80,000 (Fig. 2, lane 5) in extracts derived from yeast cells expressing a LexA-Bam fusion protein; the larger size is consistent with the predicted molecular mass of the LexA-Bam protein. Fig. 2, lane 2 shows reaction of Bam antiserum against extracts from wild-type ovaries and shows that Bam appears as a prominent band at ~Mr 55,000. This is slightly larger than the calculated molecular weight of ~Mr 50,000 (McKearin and Spradling, 1990) but comigrates with the protein produced from in vitro translation extracts (Fig. 2, lane 3). Reaction of the antisera against immunoblots containing protein extracts µ prepared from testes and 0-3 hour embryos showed that a single Mr 55,000 protein band was detected (data not shown). The presence of Bam in testes and embryos recapitulates bam mRNA expression in those tissues (McKearin and Spradling, 1990). In addition, genetic investigations of bam mutations have documented that Bam is required for normal spermatogenesis (McKearin and Spradling, 1990). Immunolocalization characterization Incubation of anti-Bam antisera with fixed ovaries revealed that Bam could be found in two distinct cellular locations. Immunoreactive Bam was found in the cytoplasm (designated BamC) of mitotically active cystocytes and in the fusome/spec- Role of Drosophila Bag of marbles protein 2941 Fig. 3. Anti-BamF immunostaining. In the succeeding panels, the anterior end of the germarium is to the left. (A) A stack of optical sections that shows the reaction of an anti-Bam antiserum that detected both BamC and BamF protein (anti-Bam2.0). Cytoplasmic staining (BamC) is clearly visible in a cluster of 8 cystocytes (5 visible) towards the top of the micrograph (labeled no. 3) as well as fainter staining in a 2-cell cluster (no. 2) towards the bottom. Fusome-associated antigen appears as bright spherical dots at the germarium anterior end in a putative stem cell (no. 1), as a slightly elongated dot connecting the 2 cystocytes labeled no. 2, and as a branched fusome (no. 4). (B) The reaction of anti-Bam.m13 antiserum against wild-type ovaries. The germarial anterior end is marked by an arrow. This antiserum reacts strongly with fusomeassociated Bam but only weakly with cytoplasmic Bam, which is not detectable in this micrograph. The high magnification series of optical sections displays the reaction against BamF antigen in germarium region 1 and shows staining of a spectrosome in a stem cell (closed arrow) and most of the fusome of an 8-cystocyte cluster (open arrow). (C) A wild-type germarium double stained with antiSpectrin and anti-Bam.m13 antisera. Spectrin staining is red while Bam staining is green. The region of overlap, which is the spectrosomes and fusomes, are therefore yellow. This micrograph was taken at approximately the same magnification as A. (D) A stack of 21 optical sections of a wild-type germarium plus a stage 2 egg chamber stained with anti-Bam.m13. Cytoplasmic Bam, as detected with this particular antiserum, is labeled BamC (arrow). The faint, string-like staining in the lower right corner of the micrograph are fusome remnants that remain in a stage 2 egg chamber that has emerged from the posterior end of the germarium. (E) The reaction of the antiserum is specific for Bam. Ovaries (Ov and arrows) from bam null mutant females (bam86 flies) were reacted in the same tube as wild-type ovaries with the anti-Bam.m13 antiserum used in D. All reaction against ovarian antigens was eliminated in bam86 ovaries. (F) Reaction of anti-BamF antiserum against a wild-type testis; again fusomes are strongly detected. The spermatocyte fusomes are thinner than those found in cystocytes and the bulbous ends tend to be more exaggerated. Note also that Bam antigen appears in many small dots that are not associated with formal fusomes. The striated structure in the center of the image (arrow) is a bundle of elongated sperm that fills the lumen of the testis; fluorescent signal from these sperm tails represents background level staining. trosome (designated BamF) in all germ cell in the germarium and in nurse cells of young egg chambers. Independently derived antisera reacted with antigen at both subcellular locations although the relative strengths of immunoreactivity varied. Antisera specificity for Bam in both cellular compartments was confirmed by demonstrating that cytoplasmic and fusome staining was eliminated in ovaries from females that were homozygous for a null allele of bam, bam86 (Materials and Methods). In addition, both staining patterns were eliminated when antisera were preadsorbed with affinity-purified histidine-tagged Bam produced in E. coli (see Material and Methods). Preincubation of the same antisera with an irrelevant affinity-purified histidine-tagged protein had no effect on anti-BamC or anti-BamF staining. Our current data about BamC and BamF do not allow us to determine if proteins found in these cellular locations represent covalently modified isoforms of Bam or differential localization of a single form of the protein. We will describe the distinct immunocytochemical staining patterns separately. Fig. 3A shows reaction of Bam antiserum with a wild-type germarium. Bam was detected in the spectrosome of a probable stem cell (1) and the antiserum stained both BamC and BamF in cystocyte clusters 2 and 3. For example, in the pair of cystocytes labeled ‘2’ in the micrograph, Bam was detected as a halo of antigen distributed throughout the cytoplasm and was concentrated in the fusome that connects the two cells. Cluster 3 (probably an 8-cell cyst) contained a high concentration of BamC and the intensity of staining in the cytoplasm obscured the signal from fusome-associated Bam. Finally a branched fusome was stained (4) in a cluster of cystocytes in germarial region 2a. Note that BamC protein was no longer found in these cystocytes. A more complete analysis of BamF protein is presented in Fig. 3B-F using an antiserum that recognizes BamF strongly but BamC only weakly. High magnification optical sections through the anterior of a wild-type germarium showed that BamF was recognized in a probable stem cell spectrosome (Fig. 3B, closed arrow) and the fusome of an 8-cell cyst (Fig. 3B, open arrow). Fusome branches terminated in an intensely stained knob within the cytoplasm of each cystocyte. Confirmation that the structure stained by anti-Bam.m13 in wild-type germaria was the fusome was obtained by co-localization of staining with anti-Spectrin antibodies (Fig. 3C). In Fig. 3C, a wild-type germarium has been stained with both anti-Spectrin (shown in red) and anti-Bam (shown in green) antisera. All spectrosomes and fusomes in the micrograph appear yellow because Bam and Spectrin staining overlap completely in the spectrosome/fusome. Further evidence of fusome recognition by Bam antibodies was obtained by noting that reaction of anti-BamF.m13 antiserum against hts1 germ cells, which do not form fusomes (Lin et al., 1994), detected only cytoplasmic Bam antigen (data not shown). Fig. 3D presents 21 superimposed optical sections through a wild-type germarium stained with anti-Bam.m13. Fusomeassociated Bam was detected in all cystocytes throughout the germarium; remnants of the fusome can be seen in individual egg chambers as mature as stage 2 (bottom right of Fig. 3D). Staining appeared most intense at the knob-like ends of the fusome branches, which may reflect greater accessibility of the antigen at those termini or may be due to an enrichment of Bam at these sites. Staining of BamC appeared as a diffuse cloud of antigen (arrow labeled BamC) and was confined to cystocytes in germarial region 1. Fig. 3E shows reaction of the anti-Bam.m13 antisera against bam86 ovaries to demonstrate that immunoreaction is specific for Bam. The failure to detect any signal above background can be attributed to specific loss of Bam antigen, and not simply elimination of fusomes, since we can show by alternate means that bam mutant germ cells contain fusomes (Figs 5, 6). In testes, anti-Bam antisera also reacted with cytoplasmic (not shown) and fusome-associated Bam (Fig. 3F). This is consistent with genetic results that indicated that bam+ is required for spermatogenesis and that the protein might serve the same or a closely related function in both male and female germ cells (McKearin and Spradling, 1990). Fig. 4A,B shows reaction of one of the Bam antisera (antiBam.m2) against wild-type ovaries. Anti-Bam.m2 reacts only with BamC in wild-type ovaries but recognizes fusome-associated Bam in at least three mutant backgrounds (not shown). We hypothesize that this reflects increased access of antibodies in this serum to Bam epitopes in the fusomes of those mutants; data from experiments that probe fusome structure in various mutant genotypes using Bam antisera will be published separately. 2942 D. McKearin and B. Ohlstein µ µ Fig. 4. Staining of cytoplasmic Bam in wild-type germaria. The anterior end of germarium is in the upper left and is marked by a curved arrow in A and a closed arrow in B. Anti-Bam.m2 antiserum was reacted against wild-type ovaries and prepared for confocal viewing using fluorescent secondary antibodies. (A) Two Bampositive cells; outlines of the other cells in this germarium can be seen faintly and represent background level signal. (B) Eight cystocytes in region 1 that are positive for BamC; the beginning of region 2a is marked by an open arrow. The micrograph presented in B was overexposed so that the outline of the germarium can be seen clearly. In all cases examined, BamC-positive cells were confined to germarium region 1. Cells in the anteriormost position, putative stem cells, were never positive. The specificity of anti-Bam.m2 for BamC protein in wildtype germ cells can be exploited to document clearly the distribution of cytoplasmic antigen. BamC is distributed throughout the cytoplasm of the cystocytes and is largely excluded from the nuclei of those cells. The number of Bam-positive cystocytes varied for different germaria (Fig. 4A,B) from as few as 1 positive cell (rarely) to as many as ~20. Most commonly a germarium had 6-10 positive cells. Cells that were positive for BamC were always confined to germarial region 1, the region that contains stem cells, cystoblasts and mitotically active cystocytes (see Fig. 1C). The most anterior cells in the germarium, the probable stem cells, were never positive for BamC antigen. Likewise cystocytes in region 2a and beyond, where completed 16-cell cysts are found, lacked BamC antigen. Thus, accumulation of BamC protein could be correlated with mitotically active cystocytes. Approximately 10% of germaria were negative for BamC antigen, suggesting Fig. 5. Anti-Fusome staining in bam86 mutants with anti-Hts. Anterior end of the germarium is in the upper left corner of A and B. (A) Wild-type fusomes visualized by anti-Hts antisera. The fusomestaining pattern is essentially indistinguishable from that described for anti-Bam antisera; note that anti-Hts also stains the membrane cytoskeleton of the somatic and germline cells. An example of a small dot of Hts-positive material not associated with fusome or membrane cytoskeleton is indicated by the open arrow. Closed arrow indicates the anterior end of germarium immediately adjacent to the terminal filaments. Also see Lin et al. (1994) for a description of anti-Hts immunohistochemistry. (B) The reaction of Hts antibodies against bam86 germarium; the micrograph is a projection of optical sections assembled through 5 µm of tissue. The fusomes recognized by Hts antiserum appear as dots or dumbbell-shaped fusomes (dbF). This germarium also shows an example of a highly elongated fusome (long arrow) that can sometimes be found connecting bam86 germ cells. that some germaria did not contain any cystocytes in mitotically active stages at the time of ovary dissection. Experiments following the incorporation of BrdU into wild-type germ cells (see below) and independent experiments carried out by H. Lin (personal communication) and Carpenter (1981) support the hypothesis that some germaria lack mitotically active cystocytes at any given time. Effects of bam mutation on fusome formation Lin et al. (1994) demonstrated that formation of an intact fusome was dependent on the Hts since strong mutations in the hts gene eliminated the fusome from germ cells. Since Bam was also a fusome protein, we tested the effects of elimination Role of Drosophila Bag of marbles protein 2943 of Bam function on fusome structure by reacting anti-Spc and anti-Hts antibodies against bam mutant ovaries. Anti-Hts staining of wild-type ovaries revealed that Hts is in spectrosomes and branched fusomes in germ cells and that Hts is associated with the membrane cytoskeleton in somatic cells (Fig. 5A; also Lin et al., 1994). The open arrow in Fig. 5A indicates small dots of Hts antigen that may represent aggregates of fusome proteins. Similar small dots can be found in cystocytes and spermatocytes using anti-Bam antisera. Reaction of anti-Hts (Fig. 5B) or anti-Spc (not shown) against bam86 ovaries revealed that nearly every cell contained a fusome. Most often (~75%) the organelles were spherical while ~25% of fusomes appeared as two spheres apposed so closely that they touched (i.e. dumbbell-shaped; dbF in Fig. 5B). Based on the appearance of fusomes in wild-type 2-cell cysts (for example, see cluster 2 in Fig. 4A), we suspected that dumbbell-shaped fusomes in bam86 samples probably passed through ring canals connecting two cells. Sometimes, long unbranched fusomes were found scattered throughout bam germaria; an example can be seen in Fig. 5B (arrow). Counterstaining nuclei with DAPI (not shown) or by locating nuclei as Spectrin-negative spheres in the faintly stained cystoplasm of bam germ cells (Fig 5B) revealed that such linear fusomes stretched between two cells. Older bam mutant cells occasionally were negative for anti-Hts or anti-Spc fusome staining probably reflecting eventual loss of the organelle in the oldest cells. Electron microscopy Strikingly, branching fusomes that would mark multicellular syncytia of cystocytes were not detected in bam mutant germ cells. This suggested that bam cells were unable to execute successive mitoses with incomplete cytokinesis to produce interconnected clusters of cells. However, there remained the formal possibility that bam cells divided with incomplete cytokinesis but that the fusome did not mark the event by extending branches through newly formed ring canals. Therefore we examined fusomes and ring canals in bam mutant cells in the electron microscope. A second goal of this analysis was to examine the ultrastructure of bam fusomes to determine if the organelle was normally formed. Fig. 6 shows a transmission electron microscope (TEM) micrograph of several germ cells and a fusome in germarial region 1. The fusome in Fig. 6A appears as a spherical region devoid of mitochondria adjacent to the nucleus (labeled ‘fu’ in Fig. 6B) The organelle is characterized by a tangled network of membranous material that fills its central region and by the exclusion of other cellular organelles such as mitochondria. Close examination has revealed that the fusome also excludes ribosomes such that the density of ribosomes within the fusome is only 10% that of surrounding cytoplasm (King, 1970). This aspect of fusome ultrastructure is difficult to see in wild-type cells because of interference by the membranous network. In some EM thin sections of extended and branched fusomes, the organelle has a fibrillar appearance similar to that depicted in the drawing in Fig. 1B; the biochemical composition of the fibrils is unknown. Electron micrographs of germ cells from bam86 ovaries showed cytoplasmic regions that resembled fusomes because of their spherical shape (as predicted by anti-Hts staining) and exclusion of ribosomes and organelles (Fig. 6C,D). Examina- tion of hundreds of bam germ cells in thin sections revealed that (1) most bam mutant fusomes are simple spheres and are contained within a single cell and (2) dumbbell-shaped fusomes that pass through ring canals can be identified in bam86 germ cells (see inset, Fig. 6C). Significantly, whenever a ring canal connected bam cells, a fusome filled the canal’s lumen. Thus, the relationship between ring canal formation and penetration of the canal by a fusome was preserved in bam mutant cells. On the basis of anti-Hts and anti-Spc-immunostained bam ovaries, we had concluded that branched fusomes did not form in bam cells. The fact that only single cells with spherical fusomes or pairs of cells connected by a ring canal and a fusome could be found in thin sections suggested that most bam cells were not connected to a neighboring cell and that none were connected to more than one cell. This conclusion was corroborated by serial reconstruction of thin sections of bam germaria. Stacked sections passed through 1-1.5 µm at intervals of 0.35 µm; in one case, sections that passed through 6 µm of a bam germarium were examined. All fusomes seen (>40) in these sections were either spherical and contained within one cell or passed through a single ring canal that connected a two cystocyte pair. The examination of bam fusomes in the EM allowed comparison of fusome ultrastructure to that found in wild-type germ cells. Lin et al. (1994) had previously examined fusome morphology in a hypomorphic bam mutant, bam1, and concluded that bam fusomes had normal ultrastructure. However, in bam86 mutant cells, the density of the vesicular material in the center of the fusome was greatly reduced compared to wild type (Fig. 6C). The vesicular material present in the mutant fusomes was sparsely scattered within the body of the fusome or was occasionally missing altogether. In wildtype germ cells, the density of the vesicular material in fusomes increases as cystocytes mature. Thus stem cell spectrosomes have the lowest density of vesicles of all germ cells. However, the amount of the membranous component in bam fusomes was reduced even below that seen in wild-type spectrosomes. Furthermore, bam fusomes had low vesicular density irrespective of their position in the mutant germarium. Thus aberrant fusome ultrastructure did not correlate with the age of bam germ cells. The difference between fusome structure in bam1 germ cells (Lin et al., 1994) and bam86 fusomes is probably because bam86 represents a complete loss of bam+ function while bam1 is partial loss-of-function allele (Ohlstein and McKearin, unpublished). Functional analysis by epistasis tests The experiments described above demonstrated that, like the Hts, Bam is a fusome component. Yet mutations in these two genes produced remarkably different phenotypes. Ovaries in hts− females contain egg chambers with fewer than sixteen cystocytes, frequently produce nurse cells and occasionally an oocyte (Yue and Spradling, 1992). Ovaries from bam− females, in contrast, contain a proliferating population of undifferentiated germ cells. The fact that Bam was found outside of the fusome (i.e. BamC) suggested a possible explanation for phenotypic differences; perhaps Bam’s activity is not limited to the fusome. This hypothesis was tested by making females mutant for both hts (which would genetically eliminate the fusome; Lin et al., 1994) and bam. If only fusome-associated 2944 D. McKearin and B. Ohlstein Fig. 6. EM micrographs of wildtype and bam fusomes. (A) A transmission electron microscope micrograph at 10,000× magnification of parts of several cystocytes in anterior germarial region 1. A fusome that lies between the nucleus and the plasma membrane occupies the center of the micrograph. The inset in the lower left corner of A shows a ring canal (the letter R marks the lumen of the canal; the rims of the canal lie above and below the letter R) and the accompanying fusome that passes through the canal’s opening. (B) A line drawing of the micrograph in A highlights the fusome (Fu) and several surrounding organelles. (C,D) A transmission electron microscope micrograph and accompanying line drawing of fusomes found in bam86 germ cells. One fusome can be seen in the center of the micrograph near the nucleus as a spherical region of reduced electron density. A second fusome can be seen in the upper portion of the micrograph. It appears as an oblong and is again marked by reduced electron density. The cells carrying these fusomes are a considerable distance from the germarial tip yet the fusomes are confined to single cells. Note that bam86 fusomes manifest a very sparse density of vesiculated bodies. The inset shows a high magnification view of a ring canal produced within a bam86 germ cell and the fusome that passes through the canal. The fusome seen in the inset is an example of a fusome that would be detected as a dumbbellshaped organelle by anti-Hts immunohistochemistry (see Fig. 4). Bam can be active, then hts; bam ovaries will resemble hts ovaries since this represents the fusome− phenotype. Therefore we constructed flies that carried mutant alleles of both hts and bam (Materials and Methods). Examination of the ovaries of such hts; bam double mutants revealed that they were indistinguishable from those produced by single mutant bam females. Thus, in genetic terms, bam is epistatic to hts. Stated differently, loss of bam+ function is epistatic to loss of the fusome and suggests that Bam protein is active independent of association with the fusome. A clear caveat to this interpretation is the extent to which hts mutations cause a complete loss of fusome function. Although the female sterile hts1 and hts2 alleles represent only partial loss of Hts function (Yue, Lin and Spradling, personal communication), Lin et al. (1994) showed with anti-Spc antibodies and TEM that no fusome could be detected in the germ cells of hts1 and hts2 animals. Furthermore, we confirmed by anti-Spc antibodies that no fusome could be detected in the germ cells of hts; bam86 double mutants. bam mutant germ cells are mitotically active The results of hts; bam double mutants showed that bam was epistatic to hts but did not order the timing of action of the two genes. However comparison of the phenotypes produced by mutant hts and bam alleles suggest that germ cell differentiation is disrupted earlier by loss of bam function. For example, hts cystocytes divide only a limited number of times and can make nurse cells and occasionally an oocyte while bam germ cells overproliferate and don’t produce any differentiated cell types. Several hypotheses could explain the germ cell hyperplasia that characterizes bam ovaries: (1) loss of Bam might cause germline stem cells to divide much more frequently than wildtype counterparts, (2) bam mutation might cause a limited expansion of the number of cells within a germarium that serve as stem cells or (3) most or all bam stem cell daughters might continue to divide indefinitely. To distinguish between these models, we assayed the mitotic activity of bam mutant cells by following DNA replication-dependent incorporation of the nucleotide analog, BrdU (Materials and Methods). Fig. 7 Role of Drosophila Bag of marbles protein 2945 recently completed DNA replication. Genome polyploidization cannot explain BrdU incorporation since DAPI staining of nuclear DNA and measurement of nuclear size (not shown) indicated that bam cells were diploid. Additional experiments demonstrated that the percentage of cells within a bam cyst that were labeled by BrdU was dependent on the length of time that the ovaries were incubated in the reagent before fixation. These results suggest that cells from any position within a bam cyst can accomplish mitosis and argue against the idea that only a small subset of bam cells is responsible for germ cell proliferation. DISCUSSION Fig. 7. Mitotic S-phase assayed by incorporation of BrdU. (A) The extent of incorporation of BrdU in wild-type ovaries after a 1.75 hour incubation in the reagent. In this example, cystocytes in region 1 are positive as well as nurse cells and follicle cells in more mature egg chambers positioned posterior to the germarium. Small BrdUpositive dots are scattered throughout the tissue; these may represent incorporation of the nucleotide analog into mitochondrial genomes although this hypothesis has not been tested directly. The anterior end of the germarium is in the upper left corner of the micrograph. (B) BrdU incorporation into bam cells. Mutant germ cells throughout a bam germarium and a tumorous cyst have taken up BrdU. An arrowhead marks the division between the germariuma and tumorous cyst. As in A, the anterior end of the germarium is in the upper left. shows an example of BrdU incorporation in wild-type and bam86 ovaries that were incubated together for 1.75 hours in the reagent. A cluster of BrdU-positive cystocytes can be seen in region 1 of a wild-type ovariole in Fig. 7A ; stem cells in this germarium did not take up BrdU in the course of the incubation. BrdU-positive nurse cells and follicle cells in more mature egg chambers were probably undergoing DNA replication associated with polyploidization of their genomes. Small BrdU-positive dots could also be detected. We hypothesize that these represent incorporation of the nucleotide analog into mitochondrial genomes since we could occasionally place the dots in cytoplasm when the corresponding nucleus (especially nurse cell nuclei) was also labeled (not shown). Incubation of bam mutant ovaries in BrdU produced evidence of very active DNA synthesis. An example of a bam86 germarium and tumorous egg chamber is shown in Fig. 7B and demonstrates that cells in every region of the germarium and egg chamber were actively replicating genomic DNA or had Cellular compartmentalization of Bam protein We have shown by immunocytochemistry that anti-Bam antibodies can recognize Bam in two distinct cellular compartments, the fusome (BamF) and the cytoplasm (BamC). One explanation that we have considered is that cytoplasmic Bam is a pool of protein that is depleted as the fusome expands. The preference that different antisera showed for BamC or BamF could be explained if some epitopes exposed in cytoplasmic Bam were less accessible in fusome-associated antigen. An alternative model is that cytoplasmic Bam carries post-translational modifications that distinguish it from fusome-associated Bam. Such modifications could have special significance in light of the results obtained with hts; bam double mutants which suggested that at least some aspects of Bam function do not require the fusome. However, our present data do not allow us to distinguish between the alternate models. BamF protein was detectable in stem cells despite the fact that bam transcripts were not detectable in these cells (McKearin and Spradling, 1990). Perhaps Bam in stem cells is derived from bam gene transcription in preadult germ cells or in stem cells at levels below those detectable by our methods of RNA in situ hybridization. We have preliminary evidence that bam is transcribed at very low levels in both preadult germ cells and adult stem cells since β-Galactosidase activity is detectable in both cell types from lacZ genes driven by a bam promoter (Ohlstein and McKearin, unpublished data). Bam is required to promote incomplete cytokinesis Most bam cells were single and unconnected to any neighbors, and remained mitotically active long after they were born (Fig. 7B). Occasionally, interconnected pairs of cells could be identified by the morphology of their fusomes or seen directly in EM micrographs. This mixture of single and pairs of cells could arise if most bam cell divisions were complete but a fraction produced stable pairs. If this explanation is correct, the fact that branched fusomes were not seen would suggest that an interconnected pair of bam cells became mitotically inactive or at least incapable of additional incomplete cytokineses. An alternative and perhaps simpler explanation for a mixture of single and paired cells is that all bam germ cell divisions include formation of ring canals but the canals between mitotic sisters break; pairs of cells could simply represent a steadystate level of sisters that have not yet broken apart. We believe that the examples of elongated fusomes stretched between two bam germ cells (Fig. 5B) might represent pairs of bam cells in the process of pulling apart at their ring canal. Precedence for 2946 D. McKearin and B. Ohlstein Stem cell Stem cell Cystoblast 2 cystocytes Bam required for switch from SC to Cb mitosis; promotes incomplete cytokinesis and activates fusome growth. 4 cystocytes 8 cystocytes 16 cystocytes Destruction of BamC blocks fusome growth and signals Cy withdrawal from mitotic cell cycle. such divisions has been described in Drosophila; ring canal formation and subsequent breakage accompanies divisions between wild-type spermatogenic stem cells and spermatoblasts (Hardy et al., 1979) and oogenic stem cells and cystoblasts (Carpenter, 1981). Although we cannot currently distinguish between the alternate views for how bam mutant cells divide completely, either explanation implies that Bam is required to promote stable incomplete cytokinesis during cystoblast division. How might Bam regulate incomplete cytokinesis in a cystoblast? A structural role in ring canal formation could explain how loss of Bam function could cause complete cytokinesis. However, bam germ cells can make ring canals (Fig. 6C) and anti-Bam antibodies place Bam in the cytoplasm and the fusome but not in the ring canal. Likewise, it is not likely that loss of fusome-associated Bam could account for complete cytokinesis of bam mutant cells because the fusome is not required for arrest of the contractile ring (Lin et al., 1994). Furthermore, hts; bam double mutants demonstrated that Bam does not require fusome association to exert its effects on cystoblast mitosis. Thus we postulate that BamC is responsible for promoting incomplete cytokinesis in the cystoblast. The differences between cystoblast and stem cell divisions imply that cystoblast differentiation includes coordinated changes for regulating cell division. Arrest of the contractile ring, growth of the fusome and changes in fusome components are examples of features that distinguish cystoblasts from stem cells. BamC may be required, by itself or together with other cytoplasmic partners, to signal a switch from stem cell to cystoblast mitoses; consequences of such a switch could include modifications of the contractile ring, promotion of fusome growth and delivery of membranous material to the fusome. The very sparse density of membranous network within bam fusomes suggests that this aspect of fusome assembly may be dependent on Bam function. As bam mutations are epistatic to hts, we propose that Bam’s association with the expanding fusome in the cystoblast follows its initial action in the cytoplasm. This model leaves open the question of how germ cells are signaled to withdraw from the mitotic cell cycle after four rounds of division. If BamC is required to carry out the first cystocyte-like division, it may be required to maintain these mitoses. Since the expression and implied turnover of BamC correlates with the mitotically active stages of cystocytes, an intriguing possibility is that degradation of BamC after the fourth cystocyte division blocks further mitosis. Perhaps Fig. 8. This figure summarizes how Bam might be required during the germ cell lineage to regulate the switch from stem cell mitoses to cystoblast mitoses. Our model also proposes that Bam is required to continue cystocyte divisions and that degradation of the BamC protein in completed cysts could stop further divisions. BamC-dependent functions, such as the delivery of membranous material to the fusome, are necessary for fusome growth and other aspects of cystocyte divisions. Fig. 8 presents features of this model in the context of the germ cell lineage. The marked disappearence of BamC from postmitotic cystocytes suggests that the protein’s cytoplasmic half-life might be regulated. We have noted previously that Bam has a PEST domain near the protein’s C-terminal end (McKearin and Spradling, 1990). Similar domains have been shown in several instances to be essential for rapid protein turnover (Rogers et al., 1986; Salama et al., 1994; Belvin et al., 1995); we are currently testing if this motif destabilizes BamC in cystocytes. Bam is probably not a primary germline sex determination factor Several genes that mutate to produce tumorous egg chambers have been shown to affect germ cell sex determination (Pauli and Mahowald, 1990; Steinmann-Zwicky, 1992; Pauli et al., 1993). To what extent the tumorous egg chamber phenotype predicts a gene involved in regulating germ cell sex determination remains an open question. As a representative of the tumorous egg chamber phenotype bam does not seem well positioned to serve as a germline sex determination factor. The bam gene does not express sexually dimorphic forms of bam mRNA (McKearin and Spradling, 1990), bam mutants express the sex appropriate form of several sexually dimorphic markers such as Sxl (Bopp et al., 1993), otu (Sass et al., 1995) and orb (McKearin and Christerson, 1994) and bam mutants are not rescued by constitutive expression of Sxl (McKearin and Christerson, 1994). The data presented in this paper identify Bam as a component of a germ-cell-specific organelle found in both males and females. Furthermore, our data suggest a role more consistent with regulating a choice between types of cell divisions rather than a direct involvement in decisions of sex determination. Perhaps the tumorous egg chamber phenotype is more a manifestation of blockage of germ cell differentiation than of germline sex determination specifically. Thus germline sex determination would be one of the steps that germ cells need to complete to progress beyond the earliest stages of differentiation. The authors thank Dr Alasdair MacDowall for expert assistance with the preparation and viewing of ovaries in the electron microscope. The authors also extend appreciation to Lynn Cooley for generous assistance in preparing anti-Bam antibodies and Haifan Lin and Allan Spradling for generously sharing data and reagents prior to publica- Role of Drosophila Bag of marbles protein 2947 tion. D. Branton graciously provided polyclonal anti-Spc antisera. The authors thank H. Krämer for his patient assistance on the Biorad MRC1000 for the double exposure in Fig. 4C. Mary Kuhn is responsible for the drawings in Fig. 6 and her artistic efforts, as well as very capable technical assistance, are greatly appreciated. S. Wasserman, F. Katz, L. Avery, L. Cooley, A. Spradling, members of McKearin laboratory and anonymous reviewers provided valuable comments on the manuscript. D. M. also thanks A. Carpenter, A. Spradling and H. Lin for useful discussions about the Drosophila fusome and L. Cooley for conversations about numerous oogenesis-related topics. The work described in this manuscript was supported by NIH Training Grant T32-GM08014 to B. O. and NIH award RO1-GM45820 to D. M. REFERENCES Belvin, M. P., Jin Y. and Anderson K. V. (1995). Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 9, 783-793. Bopp, D., Horabin, J. I., Lersch, R. A., Cline, T. W. and Schedl, P. (1993). Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118, 797-812. Carpenter, A. T. C. (1981). EM autoradiographic evidence that DNA synthesis occurs at recombination nodules during meiosis in Drosophila melanogaster females. Chromosoma 83, 59-80. Christerson, L. and McKearin, D. M. (1994). orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes Dev. 8, 614-628. Cooley, L., Kelley R. and Spradling A. C. (1988). Insertional mutagenesis of the Drosophila genome with single P elements. Science 239: 1121-1128. Gönczy, P. (1995). Towards a molecular genetic analysis of spermatogenesis in Drosophila. Ph. D. thesis. The Rockefeller University. Gondos, B. (1987a). Comparative studies of normal and neoplastic ovarian germ cells: 1. Ultrastructure of oogonia and intercellular bridges in the fetal ovary. Int. J. Gynec. Path. 6, 114-123. Gondos, B. (1987b). Comparative studies of normal and neoplastic ovarian germ cells: 2. Ultrastructure and pathogenesis of dysgerminoma. Int. J. Gynec. Path. 6, 124-131. Hall, P. A. and Watt, F. M. (1989). Stem cells: the generation and maintainance of cellular diversity. Development 106, 619-633. Hardy, R. W., Tokuyasu, K. T., Lindsley, D. L and Garavito, M. (1979). The germinal proliferation center in the testis of Drosophila melanogaster. J. Ultrastruct. Res. 69, 180-190. Hartwell, L. H. and Weinert, T. A. (1989). Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629-634. Horvitz, H. R. and Herskowitz, I. (1992). Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell 68, 237-255. King, R. C. (1970). In Ovarian Development in Drosophila melanogaster. New York: Academic Press. Lee, J. K., Coyne, R. S., Dubreuil, R. R., Goldstein, L. S. B. and Branton, D. (1993). Cell shape and interaction defects in a-Spectrin mutants of Drosophila melanogaster. J. Cell Biol. 123, 1797-1809. Lin, H., Yue, L. and Spradling, A. C. (1994). The Drosophila fusome, a germline specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120, 947-956. Lin, H. and Spradling, A. C. (1995). Fusome asymmetry and oocyte determination in Drosophila. Devel. Genet. 16: 6-12. Lindsley, D. L. and Zimm, G. G. (1992). In The genome of Drosophila melanogaster. San Diego: Academic Press. Lynch, R. G. (1995). Differentiation and cancer: the conditional autonomy phenotype. Proc. Natl. Acad. Sci. USA 92, 647-648. Mahowald, A. P. and Kambysellis, M. P. (1980). Oogenesis. In Genetics and Biology of Drosophila 2d. (eds. M. Ashburner and T. R. F. Wright), pp. 141223. New York: Academic Press. Maziarski, S. (1913). Sur la persistance des residus fusariaux pendant les nombreusesgenerations cellulaires au cours de l’ovogenese de Vespa vulgaris. Arch Zellf., Leipzig 10, 507-532. McKearin, D. M. and Spradling, A. C. (1990). bag-of-marbles; A Drosophila gene required to initiate male and female gametogenesis. Genes Dev. 4, 2242-2251. McKearin, D. M. and Christerson, L. (1994). Molecular genetics of early germ cell differentiation during Drosophila oogenesis. In CIBA Foundation Symposium; Germline Development. (eds. J. Marsh and J. Goode), pp. 210222. New York: John Wiley and Sons Ltd. Pauli, D., A. P. Mahowald. (1990). Germ line sex determination in Drosophila. Trends in Genetics 6, 259-264 Pauli, D., Oliver, B. and Mahowald, A. P. (1993). The role of the ovarian tumor locus in Drosophila-melanogaster germ-line sex determination. Development 119, 123-134. Robinson, D. N., Cant, K. and Cooley, L. (1994). Morphogenesis of Drosophila ovarian ring canals. Development 120, 2015-2025. Rogers, S., Wells R. and Rechsteiner M. (1986). Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234, 364-368. Salama, S. R., Hendricks, K. B. and Thorner, J. (1994) G1 cyclin degradation: the PEST motif of yeast Cln2 is necessary, but not sufficient, for rapid protein turnover. Mol. Cell Biol. 14, 7953-7966. Sass, G. L., Comer, A. R. and Searles, L. L. (1995). The Ovarian Tumor Protein isoforms of Drosophila melanogaster exhibit differences in function, expression and localization. Dev. Biol. 167, 201-212. Spradling, A. C. (1993a). Developmental genetics of oogenesis. In Drosophila Development (eds. M. Bate and A. Martinez-Arias), pp. 1-70. Cold Spring Harbor, NY: Cold Spring Harbor Press. Spradling, A. C. (1993b). Germline cysts: Communes that work. Cell 72, 649652. Steinmann-Zwicky, M. (1992). How do germ cells choose their sex? Drosophila as a paradigm. Bioessays 14, 513-518. Storto, P. D. and King, R. C. (1989). The role of polyfusomes in generating branched chains of cystocytes during Drosophila oogenesis. Dev. Genet. 10, 70-86. Telfer, W. H. (1975). Development and physiology of the oocyte-nurse cell syncytium. In Advances in Insect Physiology, Vol. 11. (eds. J. E. Treherne, M. J. Berridge and V. B. Wigglesworth), pp. 223-320. New York: Academic Press. Yue, L. and Spradling, A. C. (1992). hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 6, 2443-2454. (Accepted 13 June 1995)