* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Orbitals Package Examples Introduction Initialization
Orchestrated objective reduction wikipedia , lookup
Quantum computing wikipedia , lookup
Bell's theorem wikipedia , lookup
Scalar field theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
History of quantum field theory wikipedia , lookup
Atomic theory wikipedia , lookup
Quantum teleportation wikipedia , lookup
Measurement in quantum mechanics wikipedia , lookup
Renormalization wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Coherent states wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Quantum machine learning wikipedia , lookup
Many-worlds interpretation wikipedia , lookup
Quantum key distribution wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Quantum group wikipedia , lookup
EPR paradox wikipedia , lookup
Renormalization group wikipedia , lookup
Path integral formulation wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Probability amplitude wikipedia , lookup
Canonical quantization wikipedia , lookup
Quantum state wikipedia , lookup
Particle in a box wikipedia , lookup
Coupled cluster wikipedia , lookup
Spherical harmonics wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Hidden variable theory wikipedia , lookup
Hartree–Fock method wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
Electron configuration wikipedia , lookup
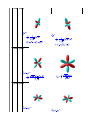
Orbitals Package Examples David A. Harrington, Chemistry Department, University of Victoria, Canada. [email protected] http://web.uvic.ca/~dharr Introduction This worksheet gives some examples of using the Orbitals Package. The Orbitals package evaluates, plots and calculates orbitals, overlap integrals, and atomic four-electron integrals for hydrogenic or Slater-type orbitals. Commands usually take either symbolic or numeric inputs to produce either formulas or numbers. For example, asking for a hydrogen orbital for quantum numbers and orbital exponent Z/n = 1 gives the 1s orbital in terms of coordinates ( ), but leaving n, l, m Documentation and references are given in the help pages, which are accessible after running the command "with (Orbitals)" in the initialization section of this worksheet. The help pages are accessed using the command ?Orbitals, or are found under "Physics/Orbitals" in the help browser. Instructions for setting up the package are given in the initialization section. Initialization Let's get started. Hit enter on the command lines to execute them, or use the !!! icon on the toolbar to execute the whole worksheet. > To set up the package, copy the files Orbitals.help and Orbitals.mla to a directory of your choice, such as "C:/Maplelibs", and make this directory known to Maple by adding it to the libname variable. Since libname usually points already to the current directory (for more recent versions of Maple), you can just copy these files to the same directory as this document. If you are accessing this document through the help system, the package is already loaded and you do not need to do anything. If you are using a directory other than the current one, uncomment the following line by removing the # and change it appropriately. > And now load the package. The commands in the package are listed. > For more details about loading the package, using it with earlier versions of Maple or modifying the source code, see the file readme.txt. Working with and plotting orbitals is the spherical harmonic or angular part of an orbital, where l is the angular momentum (azimuthal) quantum number and m is the magnetic quantum number. is the angle with the z axis in spherical coordinates and is the angle around the z axis in spherical coordinates. These angles follow the quantum mechanics convention, used here and in the VectorCalculus package, which is different from the math convention used in the rest of Maple. A orbital has and and an angular part that is the spherical harmonic worksheet makes liberal use of atomic variables to make nice looking variables such as (This , check "Atomic Variables" in the view menu to highlight these.) The function cartesian converts the spherical harmonic to the usual form in terms of x, y, z and r (use the function fullcartesian to remove the last r) > (3.1) The plots of orbitals usually seen are just plots of the squares of their angular parts (for contour plots of the wavefunction with both radial and angular parts, see below). Recall again that Maple's ( ) is ( ) in quantum mechanics, so put before in the plot command. A useful way to color these is by phase. Since this spherical harmonic is real, the phase simply indicates the sign: red for positive > Only the spherical harmonics with m = 0 are real. For example, for > we have (3.2) The square of the absolute value can be plotted in the same way as above. The colors now show > It is conventional to use only real orbitals, which can be achieved by taking linear combinations of the complex ones. For example, the and orbitals are real linear combinations of the complex spherical harmonics and . Use realY to achieve this. gives gives (A complete list of all orbitals up to f is given at the end of this worksheet.) > (3.3) Now put in the radial part as well. The radial part of a 3p hydrogen orbital (in atomic units) is given by the following call to HYDr. The arguments are the quantum numbers n and l, the orbital exponent /n ( = 1/n for hydrogen), and the name of the radial co-ordinate. > (3.4) We can do Slater type orbitals (STO) as well. > (3.5) Plot them together - the STO (blue) has no node > If is the Bohr radius, then in SI units, the hydrogenic radial function is > (3.6) Now the complete 3 orbital is the product of the radial and angular parts. Do it explicitly by multiplying the radial function by the spherical harmonic or use the built-in function HYD with arguments > (3.7) (3.7) Plot of a contour surface of the square of the complete 3 wavefunction, i.e., a probability surface, though it is non-trivial to decide what probability it encloses (here 50%). > The general formula for the hydrogen orbitals in SI units > (3.8) Plots of rigid rotor wavefunctions The spherical harmonics are also the rigid rotor wavefunctions, or the wavefunctions for the particle on a sphere. Plot the phase on the surface of the unit sphere, using a color code for the phase. Here is the (l,m)=(4,2) case. The nodes around two lines of latitude are easily seen. The colors run twice through the spectrum as one traverses azimuthally (along a latitude), consistent with m = 2. > Now do the probability density (square of the magnitude of the wavefunction) on a grayscale plot for the same wavefunction. Darker is higher probability density. > Overlap Integrals First the general formula. STOr means use the Slater Type Orbital radial function (could also use HYDr) and are the principal quantum number, angular momentum quantum number, and orbital exponent of orbital 1; likewise for orbital 2; m m quantum number of the two orbitals. R is the distance between nuclei. STOr or HYDr > (5.1) (5.1) Try a numerical one. Two 2 orbitals along the z axis 1 atomic unit apart. > (5.2) > 0.9274864666 Here is the overlap as a function of distance. (5.3) > (5.4) > Variational calculation for Helium In the simplest variational treatment of Helium, we use a configuration with the orbital exponent zeta ( ) as a variational parameter. The Hamiltonian is , and can be rearranged to , with corresponding energy . We evaluate these one at a time. ). Therefore the same energy as a 1s orbital except that Z . (aside - finding the energy of a 1s orbital from the wavefunction) he Hamiltonian is . The enegy may be found from has . > (6.1.1) > (6.1.2) The energy is the same as . > (6.1) The next term has energy and the integral can be evaluated straightforwardly: > (6.2) (6.2) The next term has the same value > (6.3) The energy for the last term is the coulomb integral > (6.4) Adding up all the energy terms, we find the total energy > (6.5) this we just solve for when the derivative dE energy gives the minimum energy. > (6.6) This result may be compared to the experimental energy -2.9033 a.u. Angular parts of orbitals Here are the angular parts of all the real orbitals up to the f orbitals. Plots are of the squares of the angular parts, with lobes red for positive angular parts, cyan for negative. Leftmost orbital is , rightmost is . (To see the code, check "show input" under table/properties.). Run the table with !!! to show the orbitals. Note that there are two common sets of real f orbitals in use, depending on which linear combinations of the eigenfunctions are chosen. The other set (not shown) is more useful for situations with cubic symmetry (see F.A. Cotton, Chemical Applications of Group Theory, 3rd ed., Wiley, NY, 1990, Appendix IV). l |m| 0 (7.1)0 (7.2) (7.1) (7.2) (7.3) 1 (7.4)0 (7.5) (7.3) (7.4) (7.5) (7.6) 1 (7.7)1 (7.8) (7.9) (7.10) 2 (7.11)0 (7.12) (7.9) (7.10) (7.11) (7.12) (7.13) 2 (7.14)1 (7.15) (7.16) (7.17) 2 (7.18)2 (7.19) (7.16) (7.17) (7.18) (7.19) (7.20) (7.21) 3 (7.22)0 (7.23) (7.24) 3 (7.25)1 (7.26) (7.24) (7.25) (7.26) (7.27) (7.28) 3 (7.29)2 (7.30) (7.31) (7.32) 3 (7.33)3 (7.34) (7.35) (7.36) (7.35) (7.36) References References used in developing the package functions are given in the help page ?Orbitals,references. The variational calculation for Helium is given in many quantum texts, e.g., D.A. McQuarrie, Quantum Chemistry, University Science Books, 1983, or I.R. Levine, Quantum Chemistry, 6th Ed., Pearson Education, 2009, sec. 9.4. Conclusions The Orbitals package is useful for plotting and manipulating atomic orbitals. Numerical results are available by specifying all parameters, but symbolic arguments generally lead to general formulas. If one or two parameters are left symbolic, plots readily show the variation with the parameter(s), e.g., overlap integrals as a function of internuclear distance. Legal Notice: The copyright for this application is owned by the author(s). Neither Maplesoft nor the author are responsible for any errors contained within and are not liable for any damages resulting from the use of this material. This application is intended for non-commercial, non-profit use only. Contact the author for permission if you wish to use this application in for-profit activities.