* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunization 5
Marburg virus disease wikipedia , lookup
Tuberculosis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Swine influenza wikipedia , lookup
Influenza A virus wikipedia , lookup
Bioterrorism wikipedia , lookup
Gastroenteritis wikipedia , lookup
Poliomyelitis eradication wikipedia , lookup
Rotaviral gastroenteritis wikipedia , lookup
Hepatitis B wikipedia , lookup
Poliomyelitis wikipedia , lookup
Orthohantavirus wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Typhoid fever wikipedia , lookup
Cysticercosis wikipedia , lookup
Meningococcal disease wikipedia , lookup
Anthrax vaccine adsorbed wikipedia , lookup
Whooping cough wikipedia , lookup
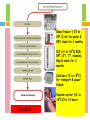
ROUTINE IMMUNIZATIONS Dr SARIKA GUPTA (MD, PhD),Assistant Professor INTRODUCTION Immunity • Ability of human body to tolerate the presence of material indigenous to the body and to eliminate foreign material • This discriminatory ability provide protection from infectious disease • Indicated by the presence of antibody to the organism causing infectious disease INTRODUCTION Vaccination versus Immunization Vaccination • Vaccination is a process of inoculating the vaccine/ antigen into the body irrespective of seroconversion Immunization • Immunization is the process of inducing immune response in an individual either humoral or cell mediated Vaccines Vaccines are whole or parts of microorganisms administered to prevent an infectious disease Biology of Vaccines Live attenuated vaccine: Consist of whole inactivated microorganisms • The immune response-similar to natural infection • Produces immunity in most of the recipients with 1 dose, except those administered orally • Active immunity may not develop because of interference from circulating antibody (Measles; OPV & rotavirus-least affected) • Could revert to its original pathogenic form, only OPV • LA viral vaccines: measles, mumps, rubella, varicella, yellow fever, rotavirus, OPV & intranasal influenza • LA bacterial vaccines: BCG & typhoid (oral) Biology of Vaccines Inactivated vaccine: Produced by growing the microorganism in culture media, inactivating with heat and/or chemicals (formalin) • Could not cause disease from infection even in an immunodeficient person • Least affected by circulating antibody • Require multiple doses • Immune response is mostly humoral, no cellular immunity • Antibody titre against IV diminish with time • Inactivated whole virus vaccines: IPV, Hep-A & rabies • Inactivated whole bacterial vaccines: Pertusis • Fractional vaccines: subunits-Hep B, influenza, acellular pertussis, HPV; toxoidsDiptheria & tetanus Biology of Vaccines Polysaccharide vaccine: A type of inactivated subunit vaccine composed of long chain of sugar molecules that make up the surface of certain bacteria • The immune response to pure PS vaccine is T-cell independent, therefore not immunogenic in children<2 years of age; pneumococcal, meningococcal & typhoid polysaccharide vaccines • Repeat dose of PS vaccine do not cause a booster response; predominant antibody produced in response to PS vaccine is IgM • Conjugation of PS vaccine with a protein molecule changes the immune response from T-cell independent to T-cell dependent- increased immunogenicity in infants & booster response; Hib A, Pneumococcal & Meningococcal conjugate vaccine Immunization schedule Ideal Immunization schedule • Epidemiologically relevant • Immunologically competent • Technologically feasible • Socially acceptable • Affordable • Sustainable For Government Programs it is cost first, efficacy next, safety last For Individual it is safety first, efficacy next, cost last National Immunization Schedule Age Vaccines Birth BCG, OPV, Hepatitis B 6 weeks DTwP1, OPV1, HepB1, Hib1±BCG 10 weeks DTwP2, OPV2, HepB2, Hib2 14 weeks DTwP3, OPV3, HepB3, Hib3 9 months Measles 16-24 months DTwP B1, OPV4, MMR, JE 5-6 years DTwP B2 10 years TT 16 years TT Vitamin A 9,18,24, 30 & 36 months IAP Immunization Time Table 2013 I: IAP recommended vaccines for routine use Age Vaccines Birth BCG, OPV0, Hep-B 1 6 weeks DTwP 1, IPV 1, Hep-B 2, Hib 1, RV5 1, PCV 1 10 weeks DTwP 2, IPV 2, Hib 2, RV5 2/RV1 1, PCV 2 14 weeks DTwP 3, IPV 3, Hib 3, RV5 3/RV1 2, PCV 3 6 months OPV 1, Hep-B 3 9 months OPV 2, Measles 12 months Hep-A 1; 2 dose after 6 months 15 months MMR 1, Varicella 1, PCV B 16-18 months DTwP B1, IPV B1, Hib B1 2 years Typhoid; revaccination every 3 years if Vi PS vaccine 4-6 years DPT B2, OPV 3, MMR 2, Varicella 2, 10-12 years Tdap/Td every 10 years, HPV IAP Immunization Time Table 2013 II: IAP recommended vaccines for HIGH-RISK children (Vaccines under special circumstances) 1. Influenza vaccine 2. Meningococcal vaccine 3. JE vaccine 4. Cholera vaccine 5. Rabies vaccine 6. Yellow fever vaccine 7. PPSV 23 High-risk category of children: Congenital/acquired immunodeficiency Chronic cardiac, pulmonary, hematologic, renal, liver disease & diabetes mellitus Children on long term steroids, salicylates, immunosuppressive or radiation therapy Cerebrospinal fluid leak, Cochlear implant, Malignancies Children with functional/anatomic asplenia/hyposplenia During disease outbreaks Laboratory personnel & health care workers Travelers Different type of adverse events following immunization Vaccine reaction Event caused/precipitated by the inherent properties of the vaccine (active component, adjuvant, preservative, stabilizer) when given correctly Program errors Event caused by an error in vaccine preparation, handling or administration Coincidental Event that happens after immunization but is not caused by the vaccine Injection reaction Event arising from anxiety about, or pain from, the injection itself rather than the vaccine Unknown The cause of the event cannot be determined Common minor vaccine reactions Vaccine Local reaction (pain, redness, swelling) Fever Irritability, malaise & non specific reactions BCG common Hib 5-15% 2-10% Adults-15% Children-5% 1-6% 10% 5-15% 50% (rash) <1% <1% Hep-B Measles /MMR OPV TT/DT/ Td 10% 10% 25% DPwT 50% 50% 60% Rare serious vaccine reactions Vaccine BCG Hep-B Reaction Suppurative adenitis, BCG osteitis, Disseminated BCGitis Anaphylaxis Measles/MMR Febrile seizures, thrombocytopenia, anaphylaxis OPV VAPP TT Brachial neuritis, anaphylaxis, sterile abscess DTP Persistent inconsolable screaming, seizures, HHE, anaphylaxis, shock JE Serious allergic reactions, neurological events YF Allergic reactions/anaphylaxis Injection site Intramuscular Injections Site Preterms & neonates Infants Anterolateral thigh (junction of middle & lower third) Toddlers & older children Deltoid or Anterolateral thigh Adolescents & adults Deltoid Subcutaneous Injections Site Infants thigh >12 months Outer triceps Intradermal Injections Site All age Left deltoid Bacille Calmette-Guerin Vaccine Supplied as a lyophilized freeze dried preparation in vacuum sealed, multidose, dark colored ampoules with normal saline as diluent In this form can be stored for 12 months at 2-8oC Light sensitive No preservative; bacterial contamination & TSS may occur if used 4-6 hours after reconstitution Site: Left shoulder; intradermal route; 26 gauze needle Dose independent of age & weight of the baby The injection site may be cleaned by saline swab A wheal of 5mm at injection site indicates successful i.d. administration of the vaccine Classical BCG reaction 2-3 weeks after BCG vaccinationdevelopment of a papule End of 5-6 weeks-papule increases to a size of 4-8mm; get converted to pustule within few days. Pustule bursts open & again get sealed off. This process continue for multiple times; at the end it dries up & a crust is formed. Crust falls off & a scar is formed by 6-12 weeks Bacille Calmette-Guerin Vaccine BCG avoided in immunocompromised children; may be given at birth in children born to HIV positive mothers BCG may be given with all vaccines on same day or at any interval with the exception of measles & MMR vaccines where a gap of 4 weeks between the two vaccines is recommended Bacille Calmette-Guerin Vaccine Recommendations for use At birth or at 6 weeks with other vaccines Catch-up vaccination with BCG: till 5 years of age Polio Vaccines OPV Two types of polio vaccines IPV With intelligent use of mOPV & bOPV, INDIA is free of polio OPV Trivalent vaccine grown in monkey kidney cell culture & stabilized with MgCl2 Light pink color-indicates right pH Most heat sensitive vaccine, Strict cold chain maintenance Given orally; two drops Contraindicated in immunodeficient children & their household contacts Polio Vaccines IPV Formaldehyde killed poliovirus, grown in monkey kidney cell or human diploid cells Contains 40, 8 & 32 D antigen units of types 1, 2 & 3 Vaccine storage: 2-8oC; Dose: 0.5 ml i.m. Seroconversion rate: 90-100% after 2 doses given after the age of 2 months & at 2 months intervals or three doses given at 6, 10 & 14 weeks Polio Vaccines Recommendations for use IPV Adoption of all IPV schedule; cessation of OPV from schedule owing to its safety issues (VAPP & cVDPVs) The phased removal of Sabin viruses has resulted in elimination of VDPV type 2 in parallel with eradication of last wild polioviruses by switching from tOPV to bOPV & mOPV The schedule that provide IPV first followed by OPV can prevent VAPP while maintaining the critical benefits conferred by OPV The birth dose of OPV is retained in countries where the risk of PV transmission is high Polio Vaccines Catch-up Schedule: IPV In children <5 years of age as three doses as 0, 2 & 6 months schedule; ensures long lasting protection In children <5 years of age, completed primary immunization with OPV, 2 doses at 2 months interval Immunodeficient children & their household contacts: IPV preferred DTwP Vaccines DTwP Compose of diphtheria & tetanus toxoids & killed whole cell pertussis bacilli adsorbed on insoluble aluminium salts (adjuvants) Vaccine storage: 2-8oC; Dose: 0.5 ml deep i.m. Immunity against all three components wanes over the next 6-12 years; therefore regular boosting needed Most adverse reactions are due to pertussis component DTwP Vaccines Absolute contraindications to ANY pertussis vaccine: History of anaphylaxis or development of encephalopathy within 7 days folllowing DTwP vaccination Precaution to future dose of DTwP : persistent inconsolable crying >3 hours, hyperpyrexia (>40.5oC), HHE within 48 hours & seizures within 72 hours of administration of DTwP Relative contraindication: progressive neurological illness DTwP Vaccine Catch-up vaccination below 7 years: three doses DTwP / DTaP at 0,1 & 6 months Catch-up vaccination above 7 years: Tdap vaccine as the first dose followed by Td vaccine as 0,1 & 6 months schedule Immunize the children recovering from diphtheria, tetanus & pertussis; Natural disease does not offer complete protection DTaP Vaccines DTaP Components of pertussis bacilli in acellular vaccines include PT with or without FHA, PRN & FIM 1,2 & 3 The DTaP vaccines score over whole cell vaccines in term of both major & minor adverse effects The absolute contraindications to DTaP vaccines are same as those for whole cell vaccine Both DTwP & DTaP must not be given in children >7 years of age TT, DT, Td & Tdap Vaccines Tdap immunization of pregnant women with a single dose of Tdap during the third trimester regardless of number of years from priorTd or Tdap vaccination. Tdap has to be repeated in every pregnancy irrespective of the status of previous immunization (with Tdap) if an adolescent girl who had received Tdap one year prior to becoming pregnant will have to take it since there is rapid waning of immunity following pertussis immunization TT in wound management History of TT doses Unknown, ≤3, immunodeficient Clean minor wounds All other wounds TT/Td YES TIG No ≥3 doses No** No ** Yes, if >10 years since last dose ***Yes, if >5 years since last dose TT/Td TIG YES YES No*** No Measles Vaccine Live attenuated vaccine Strain: Edmonstan Zagreb strain Supplied as freeze-dried in single dose or multiple dose vials with distilled water as a diluent Stored frozen at 2-80C Reconstituted vaccine: light & heat sensitive; susceptible to contamination as it has no preservative; should be kept at 2-80C, protected from light & used within 4-6 hours Measles Vaccine Dose: 0.5 ml by SC route at the age of completed 9 months In case of outbreaks, the vaccine can be given to infants as young as completed 6 months In view of 15% cases of primary vaccine failure, an additional dose of measles vaccine as MMR vaccine at the age of 15 months is required for durable & lifelong protection MMR Vaccine Supplied in lyophilized form Stored frozen for long term storage Reconstituted vaccine should be kept at 2-80C, protected from light & used within 4-6 hours Can be given with along with all others vaccine except BCG vaccine Two doses are recommended: one at the age of 12-15 months & second at 4-6 years of age Hib conjugate Vaccine Conjugated vaccine- Hib polysaccharide conjugated with a protein carrier Vaccine storage: 2-8oC; Dose: 0.5 ml i.m. Vaccine schedule: three primary doses with a booster at 18 months Vaccination started between 6-12 months: two primary doses & one booster at 18 months Vaccination started between 12-15 months: one primary dose & one booster at 18 months Children >15 months: single dose only Not recommended for NORMAL children >5 years of age Hepatitis B Vaccine Vaccine contains surface antigen of Hep B, produced by recombinant technology in yeast & adjuvanted with aluminum salts & preserved with thiomersal Available as single & multidose vials o Vaccine storage: 2-8 C; Dose: i.m 0.5 ml. for < 18 years & 1 ml in those >18 years of age Schedules: Birth, 1 & 6 months Birth, 6 & 14 weeks Birth, 6 weeks & 6 months No booster dose Catch-up vaccination: 0, 1 & 6 months Hepatitis B Vaccine Management of an infant born to Hep B positive mother: HBIg (0.5 ml) along with Hep B vaccine within 12 hours of birth using two separate syringes & on separate sites HBIg may be given upto 7 days of birth but the efficacy of HBIg after 48 hours is not known Two more doses of Hep B vaccine at 1 & 6 months of age If HBIg not available: Hep B vaccine at 0, 1 & 2 months with an additional dose between 9-12 months All such infants should be tested for HBsAg & HBsAg antibodies at the age of 9-15 months to identify carriers or non-responders Typhoid Vaccines Vi-Capsular polysaccharide vaccine The vaccine contain purified antigenic fraction of ViCapsular polysaccharide antigen of S. typhi (Virulence factor) Vaccine storage: 2-8oC; Dose: i.m 0.5 ml Not immunogenic in children below 2 years of age No immune memory; Efficacy 60% Does not interfere with the interpretation of the widal test Recommended as a single dose in children >2 years of age & revaccination every 3 years Can be safely given in immunocompromisd children Typhoid Vaccines Vi-Conjugate typhoid vaccine Conjugation of the Vi antigen with a protein carrier induces a T-cell dependent response, make it efficacious in <2 years of age & induces antibody booster response Recently a vaccine has been licensed in India where Vi antigen is conjugated with tetanus toxoid Evaluation: not satisfactory; not recommended for routine use Varicella Vaccine Derived from the Oka strain Dose is 0.5 ml by sc route; minimum infectious virus content should be 1,000 PFU Supplied in lyophilized form Should be protected from light and needs to be used within 30 minutes of its reconstitution May be given with all other childhood vaccines Recommendation: two doses of varicella vaccine given at the age of 15 months & second dose at 4-6 years VZIg: for individuals with significant contact with varicella or herpes zoster who are at high risk for severe disease Hepatitis A Vaccines Inactivated vaccine Strain is HM 175/GBM grown on MRCs human diploid cell lines The virus is formalin inactivated & adjuvanted with aluminum hydroxide Vaccine storage: 2-8oC; Dose: i.m 0.5 ml The vaccine is given in two dose schedule, 6 months apart Immunity is lifelong due to anamnestic response & no boosters are recommended in immunocompetent person Efficacy: 90-100% A liposomal adjuvanted Hep A vaccine is now available Hepatitis A Vaccines Live attenuated vaccine Strain: H2 strain of the virus attenuated after serial passage in human diploid cell line (KMB 17 cell line) Now licensed & available in India Two doses of 1 ml sc in children aged 1-15 years Rotavirus Vaccines Two live oral vaccines: human monovalent (RV1) and human bovine pentavalent rotavirus (RV5) live vaccines RV1 vaccine Strain: 89-12 grown in vero cells & contain the G1P1(8) strain Provided as a lyophilized powder The vaccine should be administered after reconstitution as 1 ml orally Vaccine storage: 2-8oC Rotavirus Vaccines RV1 vaccine Administered orally in a 2-dose schedule First dose should start at 10 weeks of age instead of 6 weeks in order to achieve better immune response The second dose can be administered at 14 weeks to fit with existing national immunization schedule RV5 vaccine This is a human bovine reassortant vaccine Consists of 5 reassortant between the bovine WC3 strain & human G1, G2, G3, G4 & P1A(8) rotavirus strain grown in vero cells Rotavirus Vaccines RV5 vaccine The vaccine is available as a liquid virus mixed with buffer Administered orally in a 3-dose schedule at 2, 4 & 6 months Vaccine storage: 2-8oC Rotavirus Vaccines First dose should be given between 6 weeks & 14 weeks 6 days of age with minimum interval of 4 weeks between the doses Immunization should not be initiated in infants 15 weeks or older All the doses of either of the vaccines should be completed within 32 weeks of age Strict schedule need to be followed as there is a potentially high risk of intussusception Preterms: Vaccination can be considered for stable & at least 6 weeks of age Rotavirus Vaccines Vaccination should be postponed in infants with acute gastroenteritis Risk vs benefits of vaccination should be considered for infants with chronic gastrointestinal disease, gut malformation, previous intussusception & immunocompromised infants Pneumococcal Vaccines Three types of pneumococcal vaccines: PCV 13, PCV 10 & unconjugated PPSV23 Pneumococcal conjugate vaccines Conjugation of PPS of varying number of serotypes with diptheria toxoid cross reactive material 197 (CRM197) proteins, protein D of non-capsulated Hib, DT & TT Pneumococcal Vaccines PCV 13 vaccines Administered by im route as a 0.5 ml dose Available in latex free, single dose prefilled syringes Can be given with all other childhood vaccines Pneumococcal Vaccines PCV 10 vaccine PCV 10 is a preservative free vaccine & adsorbed on aluminum phosphate Available in single dose prefilled syringes (0.5 ml), given by im route Primary schedule (PCV 13 & PCV 10): three doses at 6, 10 & 14 weeks with a booster at 12-15 months of age Catch-up vaccination: similar to Hib vaccine PPSV23 vaccine Recommended only for vaccination of children with certain high-risk conditions Human Papilloma Virus Vaccines HPV4 (Gardasil) & HPV2 (Cervarix) are licensed & available Manufactured by recombinant technology that produces non-infectious virus like particle comprising of HPV L1 protein, the major capsid protein of HPV HPV4 has serotypes 16, 18, 6 & 11 with aluminum adjuvant HPV2 has serotypes 16 & 18 with ASO4 as an adjuvant Either HPV4 (0, 2, 6 months) or HPV2 (0, 1, 6 months) is recommended in three dose series for females Human Papilloma Virus Vaccines Both the vaccine provide protection against cervical cancer & precancerous lesions HPV4 also protects against ano-genital warts Vaccine storage: 2-8oC Dose is 0.5 ml by im route in deltoid Catch-up vaccination till 45 years of age Should be avoided in pregnancy Contraindicated in those with history of previous hypersensitivity to any vaccine Influenza Vaccines Two types of influenza vaccine Inactivated influenza vaccine Inactivated influenza vaccine Live attenuated influenza vaccine Produced from virus grown in embryonated hen 'eggs Three types: 1. Whole virus-currently not used 2. Split product-detergent treated, purified virus 3. Subunit surface antigen- contain hemagglutinin & neuraminidase Influenza Vaccines Inactivated influenza vaccine Trivalent/monovalent Trivalent vaccine contain WHO recommended two influenza A strains (H1N1 & H3N2) & one influenza B strains Monovalent vaccine contain novel H1N1 2009 strain Storage at 2-8O C Trivalent vaccine: used in children ≥6 months Should be avoided in patients with history of GullianBarre syndrome Influenza Vaccines Live attenuated influenza vaccine Composed of the live attenuated reassortant of the three WHO recommended strains Administered as nasal spray Not available in India Cholera Vaccines Wc-rBS vaccine composed of killed vibrio cholerae 01 recombinant to subunit of cholera toxoid Oral vaccine Administered as two doses one weeks apart Protection is available after two weeks of the second dose Storage at 2-8O C Used for those aged 2 years & above Meningococcal Vaccines Unconjugated meningococcal polysaccharide vaccine Bivalent (A+C) or quadrivalent (A, C, Y & W-135) Available in lyophilized form, reconstituted with sterile water Storage at 2-8O C Indicated for children above the age of 2 years Conjugated meningococcal polysaccharide vaccine Two different types of MCVs are licensed in India quadrivalent vaccine Menactra® & a monovalent serogroup A vaccine from Serum Institute of India Meningococcal Vaccines Quadrivalent (A,C,W135,Y) MCV has diphtheria toxin as carrier protein A single dose of 0.5 mL im is recommended Efficacy: 80-85% Guillain-Barré Syndrome was noted as a possible but unproven risk in some adolescents following immunization Interference with PCV13 immune responses when MenACWY-D and PCV13 were administered simultaneously in patients with asplenia Meningococcal Vaccines Quadrivalent meningococcal polysaccharide-protein vaccine One month interval should be kept between PCV13 and MenACWY-D, and PCV13 should be administered first Monovalent serogroup A conjugate polysaccharide vaccine A lyophilized vaccine of purified meningococcal A polysaccharide covalently bound to TT acting as a carrier protein Administered as a single intramuscular injection of 0.5 mL to individuals 1-29 years of age Meningococcal Vaccines Recommendations for the use of meningococcal vaccine During disease outbreaks: most of outbreaks in India are caused by Men A, monovalent MCV should be employed in mass vaccination Children with terminal complement component deficiencies Children with functional/ anatomic asplenia/ hyposplenia (including sickle cell disease) Persons with Human Immunodeficiency Virus Meningococcal Vaccines Recommendations for the use of meningococcal vaccine Laboratory personnel and healthcare workers Adjunct to chemoprophylaxis International travelers: Students going for study abroad Hajj pilgrims Travelers to countries in the African meningitis belt Japanese Encephalitis Vaccines The vaccination against JE is not recommended for routine use, but only for individuals living in endemic areas Mouse brain derived inactivated vaccines Prepared from either the Nakayama/Beizing strain of JE virus grown in mice brain, purified, inactivated by formalin & preserved with thiomersal Given by sc route 0.5 ml in children 1-3 years & 1 ml in older children Primary immunization consists of three doses given on 0, 7 & 30 days along with boosters every 2-3 years High cost, complicated schedule & option of better vaccine – markedly declined use Japanese Encephalitis Vaccines Live Attenuated SA-14-14-2 Vaccine Contain stable neuro-attenuated strain of JE virus Dose is 0.5 ml by sc route for all ages Should not be used as an outbreak response vaccine All the children in JE endemic areas should be vaccinated First dose of the vaccine can be administered at 9 months along with measles vaccine and second at 16 to 18 months Also recommended for travelers to JE endemic areas Japanese Encephalitis Vaccines Inactivated Vero cell culture-derived SA 14-14-2 JE VaccineJEEV Recommendation is a primary schedule of 2 doses of 0.25mL for children aged 1-3 years and 2 doses of 0.5mL for children >3 years, adolescents and adults administered im on days 0 and 28 followed by a booster Japanese Encephalitis Vaccines Inactivated Vero cell culture-derived SA 14-14-2 JE VaccineJENVAC Recommendation of two doses of the vaccine (0.5 ml each) administered im at 4 weeks interval for the primary immunization series starting from 1 year of age followed by a booster Rabies Vaccines The nerve tissue vaccine- not used now Current vaccines (MTCV): PCEC vaccine, HDCV, PVCRV & PDEV Available in lyophilized form, sterile water as diluent Stored at 2-8O C Should be used within 6 hours of reconstitution Along with proper wound care & RIG, post exposure prophylaxis is effective 100% HRIG-20 IU/kg body weight ERIG-40 IU/kg body weight RIG – for category 3 wounds Rabies Vaccines PEP schedule: days 0, 3, 7, 14 & 30; sixth dose on day 90 for patients with severe debility or those who are immunosupressed If RIG not available: two doses of the vaccine can be given on day 0 Vaccines available in India for i.d. route administration are PVRV & PCEV This route should not be used for immunocompromised patients & those on chloroquine therapy Rabies Post Exposure Prophylaxis Category Description Recommended treatment I Touching of feeding of animals, licks on intact skin None if information is reliable II Nibbling of uncovered skin, minor scratches or abrasions without bleeding licks on broken skin Vaccination III Single or multiple transdermal bites or scratches, contamination of mucous membrane with saliva or exposure to bats RIG+vaccination IAP recommendations for immunization in adolescents Vaccines Schedule MMR 2 doses at 4-8 weeks interval Hepatitis B 3 doses at 0,1 & 6 months Hepatitis A 2 doses at 0 & 6 months Typhoid 1 dose every 3 years Varicella 2 doses at 4-8 weeks interval Influenza 1 dose every year JE vaccine Catch-up up to 15 years Tdap 1 dose followed by Td booster every 10 years Vaccination of person with primary immune deficiencies Category Contraindicated Vaccines Risk specificrecommended vaccine B-lymphocyte (X-linked agammagobulinemia CVID, IgA deficiency & IgG subclass deficiency) OPV, LAIV, BCG, Ty21a, YF Pneumococcal, TIV, measles & varicella T-lymphocyte (SCID, DiGeorge syndrome, Wiskott-Aldrich syndrome, ataxia-telangiectasia) All live vaccines Pneumococcal, TIV, meningococcal, Hib Comlement (Early or late, components, properdin, factor B) None Pneumococcal, TIV, meningococcal Phagocytic function (CGD, LAD, MPO) Live bacterial vaccines Pneumococcal, TIV (to decrease secondary bacterial infections) Vaccination of person with secondary immune deficiencies Category Contraindicated Vaccines Risk specific-recommended vaccine HIV/AIDS OPV, BCG, LAIV, MMR, varicella Influenza (TIV), pneumococcal, Hib, meningococcal Malignancy, transplantation, immunosuppressive or radiation therapy, Live vaccine Influenza (TIV), pneumococcal Asplenia none pneumococcal, Hib, meningococcal Chronic renal disease LAIV Pneumococcal, Influenza (TIV), Hep B Cold chain System of transporting & storing vaccines within recommended temperature from the place of manufacture to the point of administration Three main components: Trained personnel Transport & storage equipment Efficient management procedures Deep freezer (-15 to 25O C) for ice packs & OPV stock for 3 months ILR (+2 to +8OC) BCG, DPT, DT, TT, measles, Hep B stock for 3 months Cold box (+2 to +8OC) for transport & power failure Vaccine carrier (+2 to +8OC) For 12 hours Cold chain