* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Elements, Compounds and Mixtures
Size-exclusion chromatography wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
History of molecular theory wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Organic chemistry wikipedia , lookup
Boron group wikipedia , lookup
Periodic table wikipedia , lookup
Drug discovery wikipedia , lookup
History of chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical element wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Extended periodic table wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
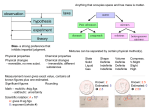
DO NOW – see board for example 1. Cut the INB template out and glue along the narrow tabs into notebook. 2. Fold each side back and add notes under each section. 3. You will refer back to this page several times as you view this presentation. © KeslerScience.com Elements and Compounds Presented by Kesler Science Essential Questions: What is the difference between elements, compounds, and mixtures? Elements and Compounds Dmitri Mendeleev • Russian chemist and inventor • Arranged the periodic table by atomic weight and similar properties • Predicted the properties of eight elements yet to be discovered Elements and Compounds Matter How is matter classified? Pure Substance Element Mixture Compound Elements and Compounds Matter • Everything around you • Anything that has mass and takes up space • Atoms and molecules are all composed of matter. • Can be solid, liquid, or gas Elements and Compounds Solid Phase of Matter • Has a definite volume and definite shape • Molecules are tightly packed. • Does not flow easily – particles cannot move/slide past one another © KeslerScience.com Elements and Compounds Liquid Phase of Matter • Has a definite volume but no definite shape • Flows easily particles can move past one another • Lots of free space between particles • Takes the shape of their containers © KeslerScience.com Elements and Compounds Gas Phase of Matter • Has no definite shape or definite volume • Lots of free space between particles • Flows easily particles can move past one another • Takes the shape and volume of its container. © KeslerScience.com Elements and Compounds Pure Substance • Has a definite and constant composition • Can be either an element or a compound • The composition of a pure substance doesn’t vary. • Ex: salt or sugar © KeslerScience.com Element H Compound H2 Compound H2O Elements and Compounds Molecule • 2 or more atoms bonded together • Represents the smallest particle in a chemical compound that can take place in a chemical reaction. • Has the same chemical properties of that element or compound. • Some molecules consist of two atoms of the same element. • Ex. O2 • Other molecules consists of two or more atoms. • Ex. (H2O) © KeslerScience.com Elements and Compounds Element • A pure substance that cannot be broken down into any other substance. • It is represented on the periodic table by a symbol. • Ex: Au is Gold © KeslerScience.com Au Au Au Elements and Compounds Compounds • A pure substance formed when one or more elements have chemically combined through a chemical reaction to form a new substance • It is represented by a formula. • Ex: NaCl - Sodium Chloride (salt) © KeslerScience.com Cl H Na O H Quick Action – INB Template 1. Cut the INB template out and glue along narrow tabs into notebook. 2. Research using the web to find the most abundant elements percentage in Earth’s crust, Earth’s atmosphere, Earth’s oceans, and Earth’s living matter. 3. Create pie charts to represent each. © KeslerScience.com Quick Action – INB Template © KeslerScience.com Elements and Compounds Counting elements in a molecule • Count the number of different capital letters or element symbols in the compound. © KeslerScience.com Quick Action – Elements and Compounds 1. Get with your table and discuss how to count the number of different elements in a compound. Formula H2O NaCl 2. Complete the chart by counting the elements and naming them. C6H12O6 NaOH © KeslerScience.com # of different elements Name of elements Quick Action – INB Template 1. Cut out the INB template and glue along the narrow tab. 2. Looking at the formula, decide if it is a compound or an element. 3. Name each element in the formula. © KeslerScience.com Elements and Compounds Mixture • A combination of many different elements • Not chemically combined • Can be separated • Ex: Trail mix © KeslerScience.com Check for Understanding Can you… Differentiate between elements, compounds, and mixtures? © KeslerScience.com