* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ch16powerpoint
Determination of equilibrium constants wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Asymmetric induction wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Process chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Marcus theory wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Hydroformylation wikipedia , lookup
Chemical reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Click chemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Rate equation wikipedia , lookup
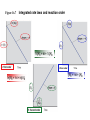
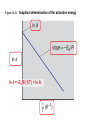
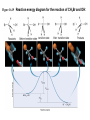
Ch #16: Kinetics • Kinetics: Rates and Mechanisms of Chemical Reactions Chemical kinetics: • Study of reaction rates, changes in concentrations of reactants or products as a function of time. Factors that influence reaction rate: 1.Concentration - molecules must collide to react; Rate α collision frequency α conc 2.Physical state - molecules must mix to collide; Smaller the particle size, greater the surface area and more the collisions. 3.Temperature - molecules must collide with enough energy to react; Rate α collision frequency α conc. Figure 16.3 The effect of surface area on reaction rate. Figure 16.4 Collision energy and reaction rate. Factors that influence reaction rate: 4.The use of a catalyst. Expressing the Reaction Rate • reaction rate - changes in the concentrations of reactants or products per unit time • reactant concentrations decrease while product concentrations increase • For a reaction A → B • Rate of reaction = -∆[A]/∆t • [A]= conc of A in mol/L • ∆=change Expressing the Reaction Rate • Units= moles per liter per second. • Mol L-1s-1 or mol/ L.s • Change I product conc is positive so the rate is Rate of reaction = ∆[B]/∆t • Rate decreases during course of reaction. • Instantaneous rate: Rate at a particular instant: considering closer values. Expressing the Reaction Rate • Use initial rate as soon as the reactants are present( no products at this time) • For a reaction aA + bB →cC+ dD, • Rate =-1/a∆[A]/∆t=1/b∆[B]/∆t==1/c∆[C]/∆t==1/d∆[A]/∆t Table 16.1 Concentration of O3 at Various Time in its Reaction with C2H4 at 303K C2H4(g) + O3(g) Time (s) - (conc A) t C2H4 O(g) + O2(g) Concentration of O3 (mol/L) 0.0 3.20x10-5 10.0 2.42x10-5 20.0 1.95x10-5 30.0 1.63x10-5 40.0 1.40x10-5 50.0 1.23x10-5 60.0 1.10x10-5 Figure 16.5 The concentrations of O3 vs. time during its reaction with C2H4 C2H4(g) + O3(g) rate = [C2H4] t - - [O3] t = C2H4 O(g) + O2(g) Figure 16.6 Tools of the Laboratory Plots of [C2H4] and [O2] vs. time. Problem 1 • P.1.2H2 (g) +O2 (g)→ 2H2O (g) Write the rate equation in terms of reactants and products. If [O2] is decreasing at 0.23 mol/L .s at what rate is [H2O] increasing? The Rate Law and its components: • Rate = k[A]m [B]n • K= rate constant(does not change as reaction proceeds) • M and n are reaction orders. • Coefficients are not related to reaction orders. • Rate constant and orders can only be found by experimental data. Determining the Initial Rate: • By performing expt and collecting data. Reaction order: • Rate=k[A]- 1st order • Rate = k[A]2- 2nd order • Rate = k[A]0- Zero order OR Rate=k(1)=k (not dependent on conc of A) Problem 2 • P.2.For each of the following reactions, determine the reaction order with respect to each reactant and the overall order from the given rate law. • 2NO(g) + O2(g) → 2NO2(g); rate = k[NO]2[O2] • CH3CHO(g) → CH4(g) + CO(g); rate = k[CH3CHO]3/2 Problem 2 • H2O2(aq) + 3I-(aq) + 2H+(aq) → (aq) + 2H2O(l); rate = k[H2O2][I-] I3- Determining Reaction Orders: • P.3.NO2(g) + CO (g) → NO (g) + CO2(g) rate=k[NO2]m [CO]n Sample Problem 16.3 Determining Reaction Order from Initial Rate Data PROBLEM: Many gaseous reactions occur in a car engine and exhaust system. One of these is NO2(g) + CO(g) NO(g) + CO2(g) rate = k[NO2]m[CO]n Use the following data to determine the individual and overall reaction orders. Experiment 1 Initial Rate(mol/L*s) Initial [NO2] (mol/L) Initial [CO] (mol/L) 0.10 0.40 0.10 2 0.0050 0.080 3 0.0050 0.10 0.20 PLAN: 0.10 Solve for each reactant using the general rate law using the method described previously. SOLUTION: rate = k [NO2]m[CO]n First, choose two experiments in which [CO] remains constant and the [NO2] varies. Sample Problem 16.3 Determining Reaction Order from Initial Rate Data continued rate 2 = rate 1 0.080 0.0050 = rate 3 rate 1 = 0.0050 0.0050 k [NO2]m2[CO]n2 k [NO2 ]m 0.40 m = 1 m The reaction is 2nd order in NO2. [NO2] 1 16 = 4m and m = 2 ; 0.10 k [NO2]m3[CO]n3 k [NO2]m1 [CO]n1 0.20 = 1 [CO]n [NO2] 2 = [CO] 3 n [CO] 1 n ; 1 = 2n and n = 0 0.10 rate = k [NO2]2[CO]0 = k [NO2]2 The reaction is zero order in CO. Determining the Rate constant: • Calculated from collected data. • Units of Rate constant depend on order. Table 16.4 An Overview of Zero-Order, First-Order, and Simple Second-Order Reactions Zero Order First Order Second Order Rate law rate = k rate = k [A] rate = k [A]2 Units for k mol/L*s 1/s L/mol*s Integrated rate law in straight-line form [A]t = k t + [A]0 ln[A]t = -k t + ln[A]0 1/[A]t = k t + 1/[A]0 Plot for straight line [A]t vs. t ln[A]t vs. t 1/[A]t = t Slope, y-intercept k, [A]0 -k, ln[A]0 k, 1/[A]0 Half-life [A]0/2k ln 2/k 1/k [A]0 Figure 16.7 Integrated rate laws and reaction order 1/[A]t = kt + 1/[A]0 ln[A]t = -kt + ln[A]0 [A]t = -kt + [A]0 Problem 5 • P.5At 1000 o C, cyclobutane (C4H8) decomposes in a first-order reaction, with the very high rate constant of 87s-1, to two molecules of ethylene (C2H4). • a) If the initial C4H8 concentration is 2.00M, what is the concentration after 0.010 s? • (b) What fraction of C4H8 has decomposed in this time? Rules for orders through graphs • • • • If a straight line is produced with the foll: Ln[reactant] vs time-1st 1/[reactant] vs time-2nd [reactant]vs time -0 order. Half Life • Half Life: Time required for the reactant concentration to reach half of its initial value. Figure 16.9 A plot of [N2O5] vs. time for three half-lives. for a first-order process t1/2 = ln 2 k = 0.693 k Problem 6 • P.6 Cyclopropane is the smallest cyclic hydrocarbon. Because its 600 bond angles allow poor orbital overlap, its bonds are weak. As a result, it is thermally unstable and rearranges to propene at 10000C via a first-order reaction: • The rate constant is 9.2s-1; (a) What is the half-life of the reaction? (b) How long does it take for the concentration of cyclopropane to reach one-quarter of the initial value? Effect of Temperature: • Temperature affects rate by affecting rate constant. • Arrhenius equ: k=Ae-Ea/RT • K=rate const, e=base of natural logs, T=absolute temp, R= Universal gas const, Ea=activation energy (minimum energy that molecules must have to react) Effect of Temperature: • • • • • • As T increases, k increases, rate increases. Ln k =ln A- Ea/R(1/T) A plot of ln k vs 1/T gives a straight line Slope=-Ea/R Y-int= ln A Ln K2/k1=-Ea/R(1/T2-1/T1) The Effect of Temperature on Reaction Rate The Arrhenius Equation k Ae Ea RT where k is the kinetic rate constant at T Ea is the activation energy R is the energy gas constant ln k = ln A - Ea/RT ln k2 k1 Ea = RT T is the Kelvin temperature A is the collision frequency factor 1 T2 1 T1 Figure 16.11 Graphical determination of the activation energy ln k = -Ea/R (1/T) + ln A Problem 7 P.7.The decomposition of hydrogen iodide 2HI(g) → H2(g) + I2(g) • has rate constants of 9.51x10-9L/mol*s at 500. K and 1.10x10-5 L/mol*s at 600. K. Find Ea. Figure 16.12 Information sequence to determine the kinetic parameters of a reaction. Series of plots of concentration vs. time Initial rates Determine slope of tangent at t0 for each plot Plots of concentration vs. time Reaction Rate constant orders (k) and actual Compare initial rate law rates when [A] Substitute initial rates, changes and [B] is orders, and concentrations Find k at held constant and into general rate law: varied T m n vice versa rate = k [A] [B] Integrated rate law (half-life, t1/2) Use direct, ln or inverse plot to find order Rate constant and reaction order Rearrange to linear form and graph Activation energy, Ea Find k at varied T Effects of concentration and temperature: • Collision Theory: Reactant particles must collide with each other. • Concs are multiplied in rate law. • Ea: Energy required to activate the molecules into a state from which reactant bonds can change into product bonds. • Only those collisions with enough energy to exceed Ea can lead to reaction. Figure 16.13 The dependence of possible collisions on the product of reactant concentrations. A B 4 collisions A B A Add another molecule of A B 6 collisions A B A Add another molecule of B A B A B A B Effects of concentration and temperature: • Rise in temp enlarges the fraction of collisions with enough energy to exceed Ea. • F= e-Ea/RT e-base of natural log, T=temp, R=gas const. • In exothermic reaction: Eaf > Ear • In endothermic reaction: Eaf < Ear • : k=Ae-Ea/RT A is the frequency factor, A=pZ, Z=collision frequency and p=orientation probability factor. Figure 16.14 The effect of temperature on the distribution of collision energies Figure 16.15 Energy-level diagram for a reaction Ea (forward) Ea (reverse) REACTANTS PRODUCTS The forward reaction is exothermic because the reactants have more energy than the products. Collision Energy Collision Energy ACTIVATED STATE Figure 16.17 The importance of molecular orientation to an effective collision. NO + NO3 2 NO2 A is the frequency factor A = pZ where Z is the collision frequency p is the orientation probability factor Transition state theory: • Transition state/activated complex: neither reactant nor product present, a transitional species with partial bonds is present. • Ea is the energy required to stretch and deform bonds in order to reach transition state. • Reaction energy diagram : TB. Pg.699 Figure 16.18 Nature of the transition state in the reaction between CH3Br and OH-. CH3Br + OH- CH3OH + Br - transition state or activated complex Figure 16.19 Reaction energy diagram for the reaction of CH3Br and OH-. Figure 16.20 Reaction energy diagrams and possible transition states. Molecularity of a reaction: • Elementary steps make up the reaction mechanism. • An elementary step is not made up of simpler steps. • Molecularity means the number of reactant particles involved in the step. Molecularity of a reaction: • • • • • In an elementary step equation coeff= order. Reaction order= molecularity A→ product Unimolecular Rate = k[A] 2A→ product Bimolecular Rate = k[A]2 A + B→ product Bimolecular Rate = k[A][B] • 2A + B→ product Termolecular Rate =k [A]2[B] REACTION MECHANISMS Table 16.6 Rate Laws for General Elementary Steps Elementary Step Molecularity Rate Law product Unimolecular Rate = k [A] 2A product Bimolecular Rate = k [A]2 A+B product Bimolecular Rate = k [A][B] 2A + B product Termolecular Rate = k [A]2[B] A Problem 8 • P.8. The following two reactions are proposed as elementary steps in the mechanism of an overall reaction: NO2Cl(g) → NO2(g) + Cl(g) NO2Cl(g) + Cl(g)→ NO2(g) + Cl(g) (a) Write the overall balanced equation. • NO2Cl(g) → NO2(g) + Cl(g) • NO2Cl(g) + Cl(g)→ NO2(g) + Cl(g) Problem 8 (a) Write the overall balanced equation. (b) Determine the molecularity of each step. (c) Write the rate law for each step Rate-Determining step: • • • • Also called as rate limiting step. It represents rate law for overall reaction. Slow reaction is a rate determining reaction. The elementary steps must add up to the overall equation. • The elementary steps must be physically reasonable. • The mechanism must correlated with the rate law. Catalysis: • Each catalyst has its own specific way of functioning. • In general a catalyst lowers the energy of activation. • Lowering the Ea increases the rate constant, k, and thereby increases the rate of the reaction • A catalyst increases the rate of the forward AND the reverse reactions. Catalysis: • A catalyzed reaction yields the products more quickly, but does not yield more product than the uncatalyzed reaction. • A catalyst lowers Ea by providing a different mechanism, for the reaction through a new, lower energy pathway