* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzymes
X-ray photoelectron spectroscopy wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Nuclear fusion wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Photoredox catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transition state theory wikipedia , lookup
Marcus theory wikipedia , lookup
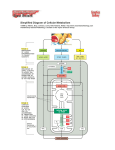
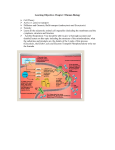
UNIT II: Intermediary Metabolism Bioenergetics and Oxidative Phosphorylation Overview • Bioenergetics describe transfer and utilization of energy in biologic systems, it makes use of few basic ideas of thermodynamics, particularly the concept of free energy • Changes in free energy (ΔG) provide a measure of energetic feasibility of a reaction & can allow prediction of whether a reaction or process can take place • Bioenergetics concerns only initial & final energy states of reaction components, not mechanism or how much time is needed for chemical change to take place • Bioenergetics predicts if a process is possible, whereas kinetics measure how fast the reaction occurs II. Free Energy - Direction & extent to which a chemical reaction proceeds is determined by the degree to which 2 factors change during reaction. - These are enthalpy (ΔH, a measure of change in heat content of reactants & products) and entropy (ΔS, a measure of change in randomness or disorder of reactants & products) - Neither of these by itself is sufficient to determine whether a chemical reaction will proceed spontaneously in the direction written - When combined math. enthalpy & entropy can be used to define a 3rd quantity, free energy (G), which predicts direction in which a reaction will spontaneously proceed Figure 6.1 Relationship between changes in free energy (G), enthalpy (H), and entropy (S). T is the absolute temperature in degrees Kelvin (°K): °K = °C + 273. III. Free energy change • Change in free energy comes in 2 forms, ΔG, ΔGº. ΔG is the more general as it predicts change in free energy & thus direction of reaction at any specified conc of reactants and products • This contrasts with change in standard free energy, ΔGº = energy change when reactants & products are at conc of 1 mol/L [ conc of protons assumed 10-7 mol/L-i.e, pH = 7] • Although ΔGº represents energy changes at these nonphysiologic conc’s of reactants & products, it is useful in comparing energy changes of different reactions. Plus ΔGº can be determined from measurement of equilibrium constant A. Sign of ΔG predicts the direction of a reaction • Change in free energy, ΔG, can be used to predict direction of a reaction at constant temp & pressure e.g., A ↔ B: 1. Negative ΔG: there is net loss of energy & reaction goes spontaneously as written. Reaction is said to be exergonic 2. Positive ΔG: net gain of energy & reaction does not go spontaneously. Reaction is endergonic, energy must be supplied to system to make reaction go 3. ΔG is zero: reactants are in equilibrium Note: when a reaction is proceeding spontaneously i.e., free energy is being lost, reaction continues until ΔG reaches zero & equil. is established Figure 6.2. Change in free energy (∆G) during a reaction. A. The product has a lower free energy (G) than the reactant. B. The product has a higher free energy than the reactant. B. ΔG of the forward and back reactions - Free energy of forward reaction (A → B) is equal in magnitude but opposite in sign to back reaction (B → A) C. ΔG depends on the concentration of reactants and products - ΔG of A → B depends on conc of reactant & product. At const temp & pressure: ΔG = ΔG º + RT ln [B]/[A] R: gas constant (1.987 cal/mol.degree) T: absolute temp (ºK) [A] & [B]: actual conc of reactant & product • A reaction with +ve ΔGº can proceed in forward direction (have a –ve overall ΔG) if ratio of products/reactants ([B]/[A]) is sufficiently small Figure 6.3. ∆G of a reaction depends on the concentration of reactant (A) and product (B). For the conversion of glucose 6-P to fructose 6-P, ∆G is negative when the ratio of reactant (A) to product (B) is large (top, panel A); is positive under standard conditions (middle, panel B); and is zero at equilibrium (bottom, panel C). D. Standard free energy change, ΔGº - ΔGº is called standard free energy change because it is equal to free energy change, ΔG, under standard conditions i.e., when reactants & products are kept at 1 mol/L. under these conditions ln [B]/[A] = 0, and ΔG = ΔGº + 0 1. ΔGº is predictive only under standard conditions: because ΔGº = ΔG. However, ΔGº can not predict direction of a reaction under physiologic conditions, because it is composed solely of constants (R, T, Keq) & is not altered by changes in product or substrate conc’s • Relationship between ΔGº and Keq: - In A → B, equilibrium is reached when no net chemical change takes place i.e., when A converted to B as fast as B is to A. In this state ratio of [B] to [A] is const. regardless of actual conc’s of the 2 cpds Keq = [B]eq/ [A]eq - If the reaction A ↔ B is allowed to go to equil. at const. temp. & pressure, then at equil. The overall free energy change (ΔG) is zero. Therefore, ΔG = 0 = ΔGº + RT ln [B]eq/ [A]eq, thus, ΔGº = - RT ln Keq ΔGº = - RT ln Keq - The above eq. allows some simple predictions: - If Keq = 1, then ΔGº = 0, reaction is at equil - If Keq > 1, then ΔGº < 0, forward reaction favored - If Keq < 1, then ΔGº > 0, back reaction favored 3. ΔGº of two consecutive reactions are additive: ΔGº’s are additive for any sequence of reactions, as are the ΔG’s • E.g., glucose + ATP → glucose-6-P + ADP (ΔGº = -4000 cal/mol) glucose-6-P → fructose 6-P (ΔGº = 400 cal/mol) ________________________________________________________ glucose 6-P + ATP → fructose 6-P + ADP (ΔGº = -3600 cal/mol) 4. ΔGs of a pathway are additive: - This property is very important in biochemical pathways through which substrates must pass in a particular direction - As long as sum of ΔGs of individual reactions is negative, pathway can potentially proceed, even if some of component reactions of the pathway have a +ve ΔG. Actual rate of reactions does depend on activity of enz’s that catalyze the reactions IV. ATP as an energy carrier • Reactions/processes that have a large +ve ΔG, e.g., moving ions against conc gradient across CM, are made possible by coupling endergonic movement of ions with a 2nd spontaneous process with a large –ve ΔG, e.g., hydrolysis of ATP • The simplest example of energy coupling in biologic reactions occurs when energy-requiring & energy-yielding reactions share a common intermediate Figure 6.4. Mechanical model of coupling of favorable and unfavorable processes. A. - - - Reactions are coupled through common intermediates Two reactions have a common intermediate when they occur sequentially so that product of 1st is substrate for the 2nd e.g., A+B→C+D D+X→Y+Z D is common intermediate & can serve as a carrier of chemical energy b/w the 2 reactions Many coupled reactions use ATP to generate a common intermediate. These reactions may involve ATP cleavage i.e., transfer of P group from ATP to another molecule. Other reactions lead to ATP synthesis by transfer of P from an energy-rich intermediate to ADP, forming ATP B. Energy carried by ATP - Adenosine (adenine + ribose) + 3 P groups - If one P removed, ADP is produced, if 2 P’s removed AMP - ΔGº of hydrolysis of ATP ~ -7300 cal/mol for each of the 2 terminal P’s. because of this large –ve ΔGº, ATP is called a high-energy phosphate cpd. Figure 6.5. Adenosine triphosphate. V. Electron Transport Chain • Energy-rich molecules, e.g., glucose, metabolized by a series of oxidation reactions ultimately CO2 & H2O. • Metabolic intermediates of these reactions donate e’s to specific coenzymes NAD+ & FAD, to form energy-rich reduced coenzymes, NADH & FADH2. • Reduced coenzymes can donate a pair of e’s each to specialized set of e-carriers, collectively called electron transport chain (ETC) • As e’s pass down ETC they lose much of their free energy. Part of which can be captured and stored by production of ATP from ADP & Pi. This process is = oxidative phosphorylation. Remainder of free energy is released as heat. Figure 6.6. The metabolic breakdown of energyyielding molecules. A. Mitochondrion - The ETC is present in inner mitochondrial membrane & is the final common pathway by which electrons derived from different fuels of the body flow to O2 Electron transport & ATP synthesis by oxphos proceed continuously in all tissues that contain mitochondria 1. Structure of mitochondria Components of ETC are located in IM. Although OM contains special pores, making it freely permeable to most ions & small molecules, IM is a specialized structure that is impermeable to most small ions, including H+, Na+, & K+, small molecules such as ATP, ADP, pyruvate, and other metabolites important to mitochondrial function Figure 6.7 Structure of a mitochondrion showing schematic representation of the electron transport chain and ATP synthesizing structures on the inner membrane. mtDNA = mitochondrial DNA; mtRNA = mitochondrial RNA. • Specialized carriers of transport systems are required to move ions or molecules across this memb. • The mitoch. IM is usually rich in protein, half of which is directly involved in e transport & oxphos • The IM is highly convoluted. Convolutions called “cristae”, greatly increase surface area of memb. 2. ATP synthase complexes - These complexes of proteins are referred to inner membrane particles and are attached to inner surface of the mitoch. IM. They appear as spheres that protrude into mitoch. matrix 3. Matrix of the mitochondrion - gel-like solution in the interior of mitoch. Is 50% protein. These molecules include E’s responsible for oxidation of pyruvate, aa’s, fatty acids (by β-oxidation), & those of TCA cycle. - The synthesis of urea & heme occur partially in matrix - In addition, matrix contains NAD+ & FAD & ADP & Pi - Matrix also contains mitochondrial DNA & RNA (mtDNA, mtRNA) & mitoch. ribosomes B. Organization of the chain - Mitoch. IM can be disrupted into 5 separate enz complexes: I,II,III,IV, & V. - Complexes I-IV each contains part of ETC, whereas complex V catalyzes ATP synthesis. - Each complex accepts or donates e’s to relatively mobile electron carriers, such as coenz. Q & cytochrome C - Each carrier in ETC can receive e’s from an edonor, & can subsequently donate e’s to next carrier in the chain - e’s ultimately combine with O2 and H+’s H2O. This requirement for O2 makes the e-transport process the respiratory chain, which accounts for greatest portion of body’s use of O2. Figure 6.8. Electron transport chain. [Note: Complex V is not shown.] C. Reactions of the ETC - With exception of coenz. Q, all members of chain are proteins. These may act as enz’s as is the case with dehydrogenases, they may contain iron as part of an iron-sulfur center, they may be coordinated with a porphyrin ring as in cytochromes, or they may contain copper, as does the cytochrome a + a3 complex 1. Formation of NADH: - NAD+ is reduced to NADH by dehydrogenases that remove 2 hydrogen atoms from their substrate. Both e’s but only one proton (i.e., a hydride ion, :H-) are transferred to NAD+, forming NADH plus a free proton, H+. 2. NADH dehydrogenase: - Free proton plus hydride ion carried by NADH are next transferred to NADH dehydrogenase, an enz complex (complex I) embedded in mitoch. IM - This complex has a tightly bound molecule of flavin mononucleotide (FMN, a coenz structurally related to FAD) that accepts the 2 H atoms (2e & 2 H+), becoming FMNH2. - NADH dehydrogenase also contains several iron atoms paired with sulfur atoms to make ironsulfur centers. These are necessary for transfer of H atoms to next member of the chain, ubiquinone (a.k.a coenz. Q) Figure 6.9. Iron-sulfur center of NADH dehydrogenase. 3. Coenzyme Q: - Co.Q is a quinone derivative with a long isoprenoid tail. It is a.k.a ubiquinone because it is ubiquitous in biologic systems - Co.Q can accept H-atoms both from FMNH2, produced by NADH dehydrogenase, & from FADH2 (complex II), which is produced by succinate dehydrogenase and acyl CoA dehydrogenase. 4. Cytochromes • Remaining members of ETC are cytochromes • Each contain a heme group made of porphyrin ring containing an atom of iron • Unlike heme groups of Hb, the cytoch. Iron atom is reversibly converted from its ferric (Fe3+) to ferrous (Fe2+) form as a normal part of its function as a reversible carrier of e’s. • e’s are passed along chain from Co.Q to cytoch b & c (complex III), & a + a3 (complex IV) 5. Cytochrom a + a3: - This cytoch complex is the only e-carrier in which heme iron has a free ligand that can react directly with molecular O2. - At this site, transported e’s, molecular O2, & free protons are brought together to produce H2O - Cytoch a+a3 (a.k.a cytochrome oxidase) contains bound copper atoms that are required for this complex reaction to occur 6. Site-specific inhibitors: - Site-specific inhibitors of electron transport have been identified & illustrated in Fig.10 - These cpds prevent passage of e’s by binding to a component of the chain, blocking the redox reaction - Therefore, all e-carriers before the block are fully reduced & those located after the block are oxidized - As electron transport & oxphos are tightly coupled, site-specific inhibition of ETC also inhibits ATP synthesis Figure 6.10 Site-specific inhibitors of electron transport shown using a mechanical model for the coupling of oxidation-reduction reactions. [Note: Figure illustrates normal direction of electron flow.] C. Release of free energy during electron transport - Free energy is released as e’s are transferred along the ETC from e-donor (reducing agent or reductant) to an e-acceptor (oxidizing agent or oxidant) - The e’s can be transferred in different forms e.g., as hydride ions (:H-) to NAD+, as H-atoms (.H) to FMN, Co.Q, & FAD, or as e’s (.e-) to cytochromes 1. - - - Redox pairs Oxidation (loss of e’s) of one cpd is always accompanied by reduction (gain of e’s) of a 2nd substance. E.g., Fig 6.11 Such redox reactions can be written as sum of two half-reactions: an isolated oxidation reaction & a separate reduction reaction (Fig. 6.11) NAD+ & NADH form a redox pair, as do FAD and FADH2. Redox pairs differ in their tendency to lose e’s. this tendency is a characteristic for a particular redox pair, & can be quantitatively specified by a constant, E◦ (standard reduction potential), with units in volts Figure 6.11. Oxidation of NADH by FMN, separated into two component redox pairs. 2. Standard reduction potential (E◦): - Standard reduction potentials of various redox pairs can be listed to range from the most negative E◦ to the most positive - The more negative E◦, the greater the tendency of the reductant member of the pair to lose e’s - The more +ve the E◦, the greater the tendency of the oxidant member of that pair to accept e’s - Therefore, e’s flow from the pair with more –ve E◦ to that with more +ve E◦ - E◦ values for some members of ETC, Fig.6.12 Figure 6.12 Standard reduction potentials of some reactions. 3. ΔGº is related to Δ E◦: - The change in free energy is related directly to the magnitude of the change in E◦ : ΔGº = -nF ΔE◦ n = # of e’s transferred (1 for cytoch., 2 for NADH, FADH2, Co.Q) F = Faraday constant (23,062 cal/volt.mol) ΔE◦ = E◦ of the e-accepting pair minus E◦ of e-donating pair ΔGº = change in the standard free energy 4. ΔGº of ATP: - The standard free energy of hydrolysis of terminal P group of ATP is -7300 cal/mol - The transport of a pair of e’s from NADH to O2 via ETC produces 52,580 cal i.e., more than sufficient energy is made available to produce 3 ATP from 3 ADP & 3 Pi (3 x 7300 = 21,900). Remaining calories are released as heat - Note: transport of a pair of e’s from FADH2 or FMNH2 to O2 via ETC produces more than sufficient energy to produce 2 ATP from 2 ADP & 2Pi VI. Oxidative phosphorylation - Transfer of e’s down ETC is energetically favored as NADH is a strong e-donor & O2 is an avid e-acceptor. But, flow of e’s from NADH to O2 does not directly result in ATP synthesis A. Chemiosmotic hypothesis (a.k.a Mitchell hypothesis) explains how free energy generated by transport of e’s by ETC is used to produce ATP from ADP + Pi 1. Proton pump: - e-transport is coupled to phosphorylation of ADP by transport of H+ across mitoch. IM from the matrix to the intermembrane space - This creates across IM an electrical gradient (with more +ve outside memb. than on the inside) & a pH gradient (outside of the memb is at lower pH than inside). - The energy generated by this H+ gradient is sufficient to drive ATP synthesis. - Thus, H+ gradient serves as the common intermediate that couples oxidation to phosphorylation 2. ATP synthase: - The enzyme complex ATP synthase (complex V) synthesizes ATP, using energy of H+ gradient generated by ETC - Note: the complex is also called ATPase, because the isolated enz. also catalyzes the hydrolysis of ATP ADP + Pi - The chemiosmotic hypothesis proposes that after H+’s have been transferred to cytosolic side of mitoch. IM, they re-enter mitoch. matrix by passing through a channel in the ATP synthase complex, resulting in synthesis of ATP from ADP & Pi and, at the same time dissipating the pH & electrical gradient Figure 6.13. Electron transport chain shown coupled to the transport of protons. [Note: Complex II is not shown.] a. Oligomycin: - This drug binds to the stalk of ATP synthase, closing H+ channel, and preventing re-entry of H+’s into mitoch. matrix - Because pH & electrical gradients can not be dissipated in presence of this drug, e-transport stops because of difficulty of pumping any more H+’s against the steep gradients - e-transport & phosphorylation are, therefore, again shown to be tightly coupled processes, inhibition of phosphorylation inhibits oxidation b. Uncoupling proteins (UCP): - UCPs occur in mitoch. IM of mammals, including humans - These proteins create a “proton leak” i.e., they allow H+’s to re-enter mitoch. matrix without energy being captured as ATP. Note: energy is released in form of heat - UCP1, a.k.a thermogenin, is responsible for activation of fatty acid oxidation and heat production in the brown adipocytes of mammals - Brown fat, unlike the more abundant white fat, wastes almost 90% of its respiratory energy for thermogenesis in response to cold, at birth, and during arousal in hibernating animals - However, humans have little brown fat (except in newborn), & UCP1 does not appear to play a major role in energy balance. - Other uncoupling proteins (UCP2, UCP3) have been found in humans, but their significance remains controversial Figure 6.14. Transport of H+ across mitochondrial membrane by 2,4-dinitrophenol. c. Synthetic uncouplers: - e-transport & phosphorylation can be uncoupled by cpds that increase permeability of mitoch. IM to H+,s - The classic e.g. is 2,4-dinitrophenol, a lipophilic H+ carrier that readily diffuses through mitoch memb. - This uncoupler causes e-transport to proceed at a rapid rate without establishing a H+ gradient, much as do UCPs. - Energy produced by transport of e’s is released as heat rather than being used to synthesize ATP. - In high doses, the drug aspirin (as well as other salicylates) uncouples oxphos. This explains the fever that accompanies toxic overdoses of these drugs B. Membrane transport systems Mitoch. IM is impermeable to most charged or hydrophilic substances. However, it contains numerous transport proteins that permit passage of specific molecules from cytosol (more correctly, intermembrane space) to mitoch matrix. 1. ATP-ADP transport: Mitoch. IM requires special carriers to transport ADP & Pi from cytosol into mitoch, where ATP can be resynthesized An adenine nucleotide carrier transports one molecule of ADP from cytosol into mitoch, while exporting one ATP from matrix back to cytosol. This carrier is strongly inhibited by the plant toxin atractyloside, resulting in depletion of intramitochondrial ADP pool & cessation of ATP production Note: a phosphate carrier is responsible for transporting inorganic phosphate from cytosol into mitoch 2. Transport of reducing equivalents: - Mitoch. IM lacks NADH transport protein, & NADH produced in cytosol cannot directly penetrate into mitoch. - However, the 2 e’s of NADH (a.k.a reducing equivalent) are transported from cytosol into mitoch using shuttle mechanisms - In glycerophosphate shuttle, 2 e’s are transferred from NADH to flavoprotein dehydrogenase within mitoch IM. This enz then donates its e’s to ETC in a manner similar to that of succinate dehydrogenase. The glycerophosphate shuttle, therefore, results in synthesis of 2 ATPs for each cytosolic NADH oxidized - This contrasts with malate-apartate shuttle, which produces NADH (rather than FADH2) in mitoch matrix, therefore 3 ATPs for each cytosolic NADH oxidized Figure 6.15. Shuttle pathways for the transport of electrons across the inner mitochondrial membrane. A. Glycerophosphate shuttle. B. Malate/aspartate shuttle. C. Inherited defects in oxidative phosphorylation - Thirteen of ~ 100 polyps required for oxphos are coded for by mtDNA, whereas remaining mitoch proteins are synthesized in cytosol & transported into mitoch. - Defects in oxphos are more likely a result of alterations in mtDNA, which has a mutation rate about 10x greater than that of nuclear DNA - Tissues with greatest ATP requirement (e.g., CNS, skeletal & heart muscle, kidney, & liver) are most affected by defects in oxphos. - Mutations in mtDNA are responsible for several diseases, including some cases of mitoch. myopathies, & Leber’s hereditary optic neuropathy, a disease in which bilateral loss of central vision occurs as a result of neuroretinal degeneration, including damage to the optic nerve. - mtDNA is maternally inherited because mitoch from sperm cell do not enter the fertilized egg. Figure 6.16. Muscle fibers from a patient with a mitochondrial myopathy show abnormal mitochondrial proliferation when stained for succinic dehydrogenase. Summary • Change in free energy ΔG predicts direction in which reaction will spontaneously proceed • If ΔG is –ve, reaction goes spont. If ΔG is +ve, reaction does not go spont. If ΔG = 0, reactants are in equil. • Change in free energy of forward & back reactions are equal but opposite in signs. • ΔGº are additive in any sequence of consecutive reactions. So, reactions or processes that have a large +ve ΔG are made possible by coupling with hydrolysis of ATP, which has a large –ve ΔGº • The reduced coenz’s NADH & FADH2 each donate a pair of e’s to a specialized set of e carriers, consisting of FMN, Co.Q, & a series of cytoch’s collectively called ETC • This pathway is present in mitoch. IM, & is the final common pathway by which e’s derived from different fuels of the body flow to O2 • The terminal cytoch a+a3, is the only cytoch capable of binding O2 • Electron transport is coupled to transport of protons (H+) across mitoch IM, from matrix to intermembrane space. • This process creates an electrical gradient & a pH gradient across mitoch IM • After H+’s have been transferred to cytosolic part of mitoch IM, they can re-enter mitoch matrix by passing through a channel in ATP synthase complex, resulting in synthesis of ATP from ADP & Pi, & at same time dissipating the pH & electrical gradient • e-transport & phosphorylation are thus said to be tightly coupled. These processes can be uncoupled by uncoupling proteins found in mitoch IM & by synthetic cpds e.g., 2,4-dinitrophenol & aspirin, all of which increase permeability of mitoch IM to H+’s. Energy produced by e-transport is released as heat rather than being used for ATP synthesis • Mutations in mtDNA are responsible for some cases of mitoch diseases e.g., Leber’s hereditary optic neuropathy Summary of key concepts for oxidative phosphorylation. [Note: Electron flow and ATP synthesis are are envisioned as sets of interlocking gears to emphase the idea of