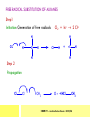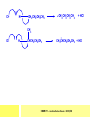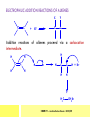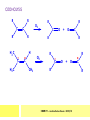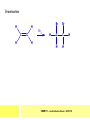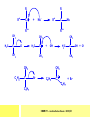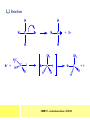* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download + ∂ - CHEM171 – Lecture Series Seven : 2012/05
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydrogenation wikipedia , lookup
Ene reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
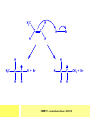
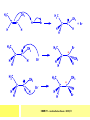
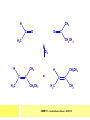
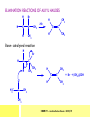
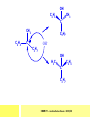
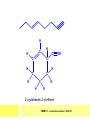
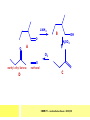
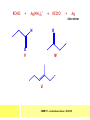
CHAPTER 7: REACTION MECHANISMS Reaction mechanisms involve the movement of electrons 1-electron 2-electrons BOND BREAKING AND BOND MAKING HOMOLYTIC FISSION: formation of free radicals X - Y → X• + Y• If we split the carbon-carbon bond in ethane homolytically, then two methyl radicals will result. CHEM171 – Lecture Series Seven : 2012/01 H3C CH 3 → 2 CH3• C2H6 → 2 CH3• HETEROLYTIC FISSION: two electrons in the bond go to one of the atoms. X Y → X+ + Y - Have a cation and an anion forming. If a carbon-carbon bond breaks in this fashion then we have a carbocation and a carbanion resulting CHEM171 – Lecture Series Seven : 2012/02 BOND FORMATION: Cl - +CH CH 3Cl 3 The chloride ion is termed a nucleophile and the carbocation is an electrophile. Reactive centre – encourages either nucleophilic or electrophilic attack. Functional groups will give rise to reactive centres within molecules. For example, if we have a ketone containing the functional group: ∂O C∂ + CHEM171 – Lecture Series Seven : 2012/03 Nu C Nu- O C O C O- Nu- This is called a concerted reaction mechanism where bonds are forming and breaking at the same time CHEM171 – Lecture Series Seven : 2012/05 CH 3 CARBOCATIONS H3C C CH 3 Cl CH 3 H3C C + Cl- CH 3 The chloride ion “leaves” the molecule and is therefore simply called a leaving group. Some carbocations are more stable than others. This comes from the so-called inductive effect. C CH 3 The inductive effect is the shifting of electron density within a σ-bond towards a positive centre in a molecule. If a substituent group is electron-donating it has a positive inductive effect and if it is electron withdrawing it is said to have a negative inductive effect. CHEM171 – Lecture Series Seven : 2012/05 FREE RADICAL SUBSTITUTION OF ALKANES Step1 Cl2 + hν 2 Cl• Initiation: Generation of free radicals: H H Cl H C H Cl H + C H H H Step 2 Propagation Cl Cl CH3 Cl + HCl CH 3 CHEM171 – Lecture Series Seven : 2012/06 Step 3 Termination For a free radical substitution to stop, free radicals must react with each other. Cl Cl Cl Cl Cl CH 3 Cl CH 3 CH 3 CH 3 C2 H6 Consider the free radical substitution of larger alkanes: CHEM171 – Lecture Series Seven : 2012/07 Cl H CH 2CH2 CH2CH3 CH 2CH2 CH2CH3 + HCl CH 3 Cl H CHCH2CH2CH3 CH 3CHCH2CH2 CH3 + HCl CHEM171 – Lecture Series Seven : 2012/08 ELECTROPHILIC ADDITION REACTIONS OF ALKENES C C + XY X Y C C Addition reactions of alkenes proceed via a carbocation intermediate. H C H H H C H H Br H C C H H H3C H + Br- CH 2Br CHEM171 – Lecture Series Seven : 2012/09 H3 C H C H C H H H H3C Br H C C H H H + Br- H C C H H CHEM171 – Lecture Series Seven : 2012/10 CH 3 + Br- H3 C CH3 C H3 C C H H Br C H H H3 C C H H H3 C C H Br- H H C H Br C H Br- * C H H H CH3 CH3 C H + Br- H H3 C CH3 H C H3 C CH3 C CH3 *C H Br CHEM171 – Lecture Series Seven : 2012/11 OZONOLYSIS R R C C R H3 C H3 C O3 R * C x C H CH3 R R C O + O C R O3 R R C* O + O R CHEM171 – Lecture Series Seven : 2012/12 x C R R CH3 H C O O C CH2CH 3 H3 C O3 H CH3 C H3 C H or C CH2CH 3 CH2CH 3 C H3 C C CH3 CHEM171 – Lecture Series Seven : 2012/13 ORGANIC REDOX CHEMISTRY The oxidation states in between +4 and –4 will be encountered for carbon compounds which contain both oxygen and hydrogen. CO2 : C in the +4 oxidation state CH4 : C in the -4 oxidation state Addition of hydrogen results in reduction Addition of oxygen results in oxidation CHEM171 – Lecture Series Seven : 2012/14 INTER-CONVERSION OF ALCOHOLS/ ALDEHYDES/ KETONES/ CARBOXYLIC ACIDS OXIDATION 1o alcohol oxidized to an aldehyde H R C OH CrO3 H H+ C O C O R H 2o alcohol oxidized to a ketone H R C R' OH CrO3 R H+ R' CHEM171 – Lecture Series Seven : 2012/15 If we treat a 1o alcohol with excess CrO3 in acid, a carboxylic acid is formed. H R C O OH CrO3/H+ R excess C OH H An aldehyde or ketone is reduced to the respective alcohols using a mild reducing agent, sodium borohydride, NaBH4. Carboxylic acids can be reduced to an aldehyde using NaBH4 or directly to the alcohol using a stronger reducing agent, lithium aluminium hydride, LiAlH4 CHEM171 – Lecture Series Seven : 2012/16 ADDITION REACTIONS OF ALKENES Hydration H3 C CH3 C C H3 C H2O/H+ H3C H OH H C C H CH 3 CH 3 Hydrogenation H H C H C H H2 catalyst H H H C C H H H CHEM171 – Lecture Series Seven : 2012/17 Bromination H H C H C H Br2 H Br Br C C H H H CHEM171 – Lecture Series Seven : 2012/18 ELIMINATION REACTIONS OF ALKYL HALIDES H H Br C C H CH 3 CH 3 H -HBr CH3 C C H CH3 Base- catalysed reaction H H C C H CH 3 OH3C C Br CH3 H CH3 C H + Br- + (CH3 )3COH C CH3 CH 3 CH 3 CHEM171 – Lecture Series Seven : 2012/19 NUCLEOPHILIC SUBSTITUTION REACTIONS OF ALKYL HALIDES Nu- + C X C Nu + X- SN1 Reactions SN1 reactions proceed via a carbocation intermediate. R R'' C R''' R X R'' C + X- R''' CHEM171 – Lecture Series Seven : 2012/20 R R'' C R + Nu- R'' C R''' R''' CH 3 H3C C CH 3 Cl H3C CH 3 C CH 3 + OH- H3C CH 3 C C3 H7 C CH 3 CH 3 C2 H5 Nu CH 3 Br C2 H5 C + BrC3H 7 CHEM171 – Lecture Series Seven : 2012/21 OH + Cl- OH C 2H5 CH 3 C2 H5 C CH3 C3 H7 C OHC3H 7 OH H3C C C2H 5 C3 H7 CHEM171 – Lecture Series Seven : 2012/22 SN2 Reactions H H H C Br H C H Br- + H3C H H - CH 3 C I H + Br - Br C H I H CH 3 Br C H CHEM171 – Lecture Series Seven : 2012/23 + IH ACIDS AND BASES Carboxylic Acids RCOOH + H2O ⇌ RCOO- + H3O+ O- O R C R O- C O O R C O CHEM171 – Lecture Series Seven : 2012/24 Alcohols R – O – H + base R – O- + base – H+ O H O- + NH2- NH 3 + H R O H+ H R O H oxonium ion CHEM171 – Lecture Series Seven : 2012/25 CONDENSATION REACTION CH3CH2COOH + CH3CH2OH CH3CH2COOCH2CH3 + H2O TERMINAL ALKYNES R – C ≡ C – H, weakly acidic and will react with a strong base such as sodium amide: R C C NH2- H R C C + NH 3 R R C C C H X R C C CH 2R + X - H CHEM171 – Lecture Series Seven : 2012/26 O R C C O C O H+ R C C C OH REAGENTS AND REACTION CONDITIONS Most organic reactions need to have the following specified. • • • • solvent temperature catalyst reagents to be used R C C R' + H2 ? CHEM171 – Lecture Series Seven : 2012/27 H H C H C R' C R R' C R R H H H C C H H R' The actual product formed depends upon the experimental conditions R C R C C R' C H H2 C Lindlar's catalyst R' 2 H2 Pd/C H C R R R' H H C C H H R' CHEM171 – Lecture Series Seven : 2012/28 R CH2 OH R CH2 CrO3 H+ OH R PCC CHO R CrO3 H+ R CHO CH2Cl2 PCC = pyridinium chlorochromate CHEM171 – Lecture Series Seven : 2012/29 COOH EXAMPLE A hydrocarbon of unknown structure has the molecular formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a Pd/C catalyst 3 equivalents of H2 react. Draw a structure for the hydrocarbon that fits the data. SOLUTION R C C R' C C C H H R' C R CHEM171 – Lecture Series Seven : 2012/30 H H C H C C CH C H H C C H C H H H 2-cyclohexen-2-ylethyne CHEM171 – Lecture Series Seven : 2012/31 EXAMPLE An unknown hydrocarbon, A, has a formula of C6H10. On catalytic hydrogenation over Pd/C it reacts with only 1 equivalent of H2. A also undergoes reaction with ozone to give a single product a dialdehyde, B. Suggest a structure for A and B. SOLUTION H C H O H H C H C H H C C H C H H H O H CHEM171 – Lecture Series Seven : 2012/32 EXAMPLE How could you prepare 2-methylhexane from an alkyl halide and an alkyne? SOLUTION H C C H + NH2- H C C + Cl 2H2 Pd/C CHEM171 – Lecture Series Seven : 2012/33 + NH3 EXAMPLE An aldehyde A (C5H10O) can be reduced by LiAlH4 to B. Treatment of B with concentrated H2SO4 gave C. Ozonolysis of C affords methanal and D (C4H8O). D gave a positive iodoform test which identifies methylketones. Identify A, B, C and D. SOLUTION O O + 3NaOI C R CH3 + 3NaOH C R CI 3 O + NaOH C R CI 3 RCOO-Na+ + CHI3 yellow ppt. CHEM171 – Lecture Series Seven : 2012/34 LiAlH 4 O O B OH H2SO4 A O3 methyl ethyl ketone D O methanal C CHEM171 – Lecture Series Seven : 2012/35 EXAMPLE A hydrocarbon A adds one equivalent of hydrogen in the presence of a Pd/C catalyst to form n-hexane. When A is reacted vigorously with KMnO4 a single carboxylic acid containing three carbon atoms is isolated. Give the structure and name of A. SOLUTION O KMnO4 O OH + HO CHEM171 – Lecture Series Seven : 2012/36 O OH CHEM171 – Lecture Series Seven : 2012/37 EXAMPLE The trans isomer of an unknown alkene U was reacted with ozone followed by Zn in water. The products of the reaction were V, C4H8O and W, C3H6O. Only W gave a positive Tollen’s test. (i) What information is obtained from the Tollen’s test? (ii) What would you see that will tell you if the test was positive? (iii) Draw a structure for the trans isomer of U and structures for, V and W SOLUTION (i) Tollen’s test is used to distinguish between an aldehyde and ketone (ii) Tollen’s reagent contains the silver ammonia ion, Ag(NH3)2+. Oxidation of the aldehyde is accompanied by reduction of silver ion to free silver – in the form of a mirror) CHEM171 – Lecture Series Seven : 2012/38 RCHO + Ag(NH3)2+ RCOO- + Ag silver mirror H O O V W U CHEM171 – Lecture Series Seven : 2012/39 EXAMPLE Compound A, C4H8, reacts with O3 to give methanal and compound B. Compound B reacts with CrO3 to give compound C. Compound B also reacts with LiAlH4 to give D. Upon treatment with 95% H2SO4 at 170oC compound D gives rise to compound E. E, when treated with HCl gives us the major product F and also a minor product G. Identify compounds A to G. SOLUTION O3 A H HCHO + B O CHEM171 – Lecture Series Seven : 2012/40 H OH CrO3 B C O O LiAlH 4 H D H2SO4 170°C E OH HCl Cl F Cl major G minor CHEM171 – Lecture Series Seven : 2012/41 Markovnikov’s rule: in the addition of an acid to a carboncarbon double bond of an alkene, the hydrogen of the acid attaches itself to the carbon that already holds the greater number of hydrogens. On addition of peroxide- get Anti-Markovnikov. Cl HCl H2O2 Cl Minor major CHEM171 – Lecture Series Seven : 2012/42