* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Coordination Chemistry
Jahn–Teller effect wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Cluster chemistry wikipedia , lookup
Oxidation state wikipedia , lookup
Hydroformylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metal carbonyl wikipedia , lookup
Bond valence method wikipedia , lookup
Spin crossover wikipedia , lookup
Stability constants of complexes wikipedia , lookup
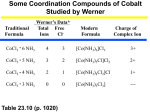
Coordination Chemistry Contents I Introduction 1 History 2 Definition 3 Nomenclature II Bonding 1 VBT & Crystal Field Theory 2 MOT & Ligand Field Theory 3 Electronic Spectroscopy 4 Magnetic Properties III Structure of Coordination Compounds IV Reactivity & Reaction Mechanisms V Descriptive Chemistry I Introduction 1 History 1827 Discovery of Zeise’s Salt (KPtCl3C2H4) The war between Alfred Werner & Sophus Jørgensen — an experimentalist versus an experimentalist seeking for insight Complex Color old name IUPAC name CoCl3·6NH3 yellow Luteo complex hexaamminecobalt(III) chloride CoCl3·5NH3 purple Purpureo complex pentaamminechlorocobalt(III) chloride CoCl3·4NH3 green Praseo complex trans-tetraamminedichlorocobalt(III) chloride CoCl3·4NH3 violet Violeo complex cis-tetraamminedichlorocobalt(III) chloride Jørgensen Stick on the idea of “valence” Co(NH3NH3Cl)3 Cl2Co(NH3NH3NH3NH3NH3Cl) ClCo(NH3NH3Cl)2 Cl2Co(NH3NH3NH3NH3Cl) By considering both precipitation of AgCl and conductivity (Table 11.1): Werner proposed: [Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 [Co(NH3)4Cl2]Cl [Co(NH3)4Cl2]Cl primary valence (formal oxidation state) secondary valence (coordination number) Werner’s experiments for the conductivity of some coordination complexes 2 Definition A “coordination compound” is consisted of two components: the metal ion (atom) and the ligand(s). A “ligand” is an ion, a molecule, or a group that is associated with the metal ion as a Lewis acid-base adduct. The “donor atom” is the atom in the ligand, that attaches to the metal ion. A ligand has only one donor atom is a “monodentate”; with two donor atoms will be “bidentate”; “tridentate” for three; “tetradentate” for four…..etc. A ligand that has more than one donor atom is of “multi-dentate”. The association mold of a multidentate is called “chelation”. A “bridging ligand” attaches to more than one metal ion. Isomerism and Structural insight Structural Isomers Hydrate Isomers [Co(NH3)4(H2O)Cl]Cl2 and [Co(NH3)4(Cl2)]Cl·H2O Ionization Isomers [Co(NH3)5(SO4)](NO3) and [Co(NH3)4(NO3)](SO4) Coordination Isomers [Co(en)3][Cr(CN)6] and [Co(CN)6][Cr(en)3] Linkage Isomers Pd(AsPh3)2(SCN)2 and Pd(AsPh3)2(NCS)2 [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+ Stereoisomers Four-Coordinate Tetrahedral TiCl4, CoCl42-, CuCl42-, Pt(PPh3)4, Geometrical Isomers Optical Isomers Lacking two perpendicular C2 symmetry Six-Coordinate Geometrical Isomers Trans and cis - forms mer and fac - forms For an octahedral complex that has six different ligands Two optical isomers Structure versus number of isomers Questions remained: Are isomers always synthesizable? Optical Isomers with Bidentate Chelation nl - nr nl and nr are the refractive index of the left and right circularly polarized light respectively [], the specific rotation is defined as left; wherein c' is the concentration and d' is the thickness of the sample c'd' MWxis molar rotation l - r kl - kr c kl and kr are the absorption coefficients of the left and right circularly polarized light respectively Asymmetric bidentate ligands chiral without carbon Tridentate Tetradentate