* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 6 Answers Energy and Life Visual Understanding Figure
Photosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Human digestive system wikipedia , lookup
Proteolysis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
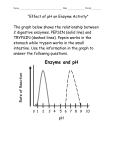
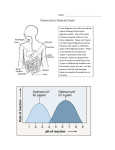
Chapter 6 Answers Energy and Life Visual Understanding Figure 6.8 1. You eat a hamburger. Salivary amylase begins to digest the carbohydrates in the bun while you are still chewing. Pepsin works in your stomach to digest the protein, and trypsin is active in your small intestine to break the bonds between specific amino acids. How does the optimum pH for pepsin and trypsin reflect this chain of events? Although the pH of your saliva is only very slightly acidic, about pH 6, the pH in your stomach is very low, about 1 to 2. However, as food passes from the stomach into the upper portion of the small intestine, chemicals are added that rapidly raise the pH to about 6, and then, over time, to about 7 or 8. Thus pepsin, with an optimum pH of 2 to 3, is active in breaking down large protein molecules in your stomach, and trypsin, with an optimum pH of 6 to 8, works best in your small intestine where it breaks bonds between particular amino acids. 2. Table 6.1 ATP comes primarily from the breakdown of glucose. If your blood glucose level drops, what sorts of problems can that cause? ATP is required to provide energy for many cellular processes. When blood glucose drops, ATP production can decrease and therefore all of these processes may be impacted. Cells require ATP for active transport mechanisms, so importation of metabolites like amino acids and sugars will be affected, as will functioning of the Na-K pump. ATP is required for many movement-related functions such as muscle contraction, flagellar movement, cell crawling, and cytoplasmic transport of vesicles and organelles, so these processes can be ultimately disrupted. Utilization of ATP produces heat as a byproduct, so thermoregulation can be a potential problem. Challenge Questions 1. Living organisms on the surface of the planet and the top layer of the ocean get their energy from the photosynthetic plants and bacteria that capture the sun’s energy and transform it to molecules that other organisms can use. At the bottom of some deep ocean trenches there are entire colonies of many types of organisms. What do they use for energy? The heated water, filled with chemicals, that comes from the deep vents provides the basis for chemosynthetic bacteria to capture energy. Other life forms then live on the bacteria, and the food web grows from there. 2. Why do you need enzymes to help your cells do their jobs? The things that cells do can be done without enzymes, but it would take a very long time. The enzymes speed things up so that foods are broken down, parts of your body are repaired and replaced, and new things are made in a timely manner. 3. What sorts of things can keep an enzyme from doing its job? If an enzyme is denatured, then its bonds are broken and it loses its shape, leaving it unable to do its job. This could happen if you had a high temperature, or if your body’s pH was not correct in the various places where it needs to change. If something is blocking an enzyme’s active site or somehow changing the shape of the active site, then the substrate molecule cannot fit. 4. After a rain your new iron fenceposts are rusting. Explain how this is an example of a redox reaction, just like the ones that occur in your body. Now can you think of a reason why blood coming from the lungs is bright red? In the presence of moisture, such as rain, iron will combine with oxygen to form the bright red compound iron oxide, which we call rust. This oxidized iron has “lost” two electrons to the oxygen atom. In your red blood cells are molecules called hemoglobin. At the center of every hemoglobin complex are four iron atoms. When the blood reaches your lungs it picks up oxygen which attaches to these iron atoms, turning your blood a brighter red color.