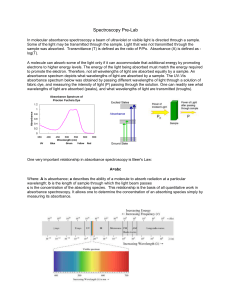
About UV-Vis Molecular Absorbance Spectroscopy
... Where: A is absorbance; a describes the ability of a molecule to absorb radiation at a particular wavelength; b is the length of sample through which the light beam passes c is the concentration of the absorbing species. This relationship is the basis of all quantitative work in absorbance spectrosc ...
... Where: A is absorbance; a describes the ability of a molecule to absorb radiation at a particular wavelength; b is the length of sample through which the light beam passes c is the concentration of the absorbing species. This relationship is the basis of all quantitative work in absorbance spectrosc ...
EP225 Lecture 31 Quantum Mechanical E¤ects 1
... The electron was identi…ed much later in 1900 by Thomson and at the time of Hertz’s experiment, it was not clear what was really carrying electric current which was the quantity measured in the experiment. It is shown schematically in Fig. 3 (a). When light is on, the tube becomes a diode with the i ...
... The electron was identi…ed much later in 1900 by Thomson and at the time of Hertz’s experiment, it was not clear what was really carrying electric current which was the quantity measured in the experiment. It is shown schematically in Fig. 3 (a). When light is on, the tube becomes a diode with the i ...
AP Chemistry
... Molecular solids Malleable Ductile Band model Alloys: substitutional, interstitial Diamonds vs graphite ...
... Molecular solids Malleable Ductile Band model Alloys: substitutional, interstitial Diamonds vs graphite ...
Electron Notes
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
... • One experiment involved the photoelectric effect, which refers to the emission of electrons from a metal when light shines on the metal. • This involved the frequency of the light. It was found that light was a form of energy that could knock an electron loose from a metal. ...
Lecture. Photoelectric Effect
... the particles emitted by the body under illumination – the same as for electrons. The effect remained unexplained until 1905 when Albert Einstein postulated the existence of quanta of light -- photons -- which, when absorbed by an electron near the surface of a material, could give the electron enou ...
... the particles emitted by the body under illumination – the same as for electrons. The effect remained unexplained until 1905 when Albert Einstein postulated the existence of quanta of light -- photons -- which, when absorbed by an electron near the surface of a material, could give the electron enou ...
Physics, Chapter 43: X-Rays - DigitalCommons@University of
... discovery of x-rays by W. C. Roentgen in 1895. While operating a gasdischarge tube, Roentgen observed that a platinum-barium cyanide screen at some distance from the tube fluoresced. He shielded the tube so that no visible radiation could reach the screen, but the fluorescence could still be observe ...
... discovery of x-rays by W. C. Roentgen in 1895. While operating a gasdischarge tube, Roentgen observed that a platinum-barium cyanide screen at some distance from the tube fluoresced. He shielded the tube so that no visible radiation could reach the screen, but the fluorescence could still be observe ...
absorbance, a - srmbiotech25
... produce more then one order of diffraction. • For instance, the second order of 400 mμ may interfere with the first order of 800 mμ. • This can be removed my employing filters in front of the entrance slit to absorb interfering radiation. ...
... produce more then one order of diffraction. • For instance, the second order of 400 mμ may interfere with the first order of 800 mμ. • This can be removed my employing filters in front of the entrance slit to absorb interfering radiation. ...
Lecture24 - Purdue Physics
... a football game, etc.) In science, the existence of atoms (different chemical ...
... a football game, etc.) In science, the existence of atoms (different chemical ...
$doc.title
... Equivalent analysis of Young’s (Two) Slits using 1st maximum, Where slit separation is the uncertainty in position (exercise) Q: “which slit does the particle (or photon) go through?” !! ...
... Equivalent analysis of Young’s (Two) Slits using 1st maximum, Where slit separation is the uncertainty in position (exercise) Q: “which slit does the particle (or photon) go through?” !! ...
First Semester Honors Chemistry Exam Review (2011
... 72. Malleability and ductility are characteristic of substances with what type of bonds? 73. What does the 218 in polonium-218 represent? 74. What equation shows the correct relationship between mass and energy? 75. Reactions that affect the nucleus of an atom are called______. 76. What is the proce ...
... 72. Malleability and ductility are characteristic of substances with what type of bonds? 73. What does the 218 in polonium-218 represent? 74. What equation shows the correct relationship between mass and energy? 75. Reactions that affect the nucleus of an atom are called______. 76. What is the proce ...
Quantum Imaging beyond the shot noise limit
... the measurement of laser intensity is uncertain, varying slightly from pulse to pulse. This noise arises from the particle (photon) nature of light, and leads to the fundamental limit in precision that we can ever achieve with a laser: shot-noise. This can be an issue in scenarios such as spectrosco ...
... the measurement of laser intensity is uncertain, varying slightly from pulse to pulse. This noise arises from the particle (photon) nature of light, and leads to the fundamental limit in precision that we can ever achieve with a laser: shot-noise. This can be an issue in scenarios such as spectrosco ...
Transcript - the Cassiopeia Project
... But of course, the atom is not always found in this lowest energy state. As there are other orbits allowed in the Bohr model, there are other, higher energy states in the quantum mechanical hydrogen atom. These states are defined primarily by the quantum number “n” that we talked about earlier. And ...
... But of course, the atom is not always found in this lowest energy state. As there are other orbits allowed in the Bohr model, there are other, higher energy states in the quantum mechanical hydrogen atom. These states are defined primarily by the quantum number “n” that we talked about earlier. And ...
Final Exam
... 3) A He-Cd laser (442 nm) is incident on a screen containing two very narrow horizontal slits separated by 180 µm. A fringe pattern appears on a screen held 1.2 m away. a) What is the distance from the central axis to the first zero of the light intensity? b) How far in mm from the central axis is t ...
... 3) A He-Cd laser (442 nm) is incident on a screen containing two very narrow horizontal slits separated by 180 µm. A fringe pattern appears on a screen held 1.2 m away. a) What is the distance from the central axis to the first zero of the light intensity? b) How far in mm from the central axis is t ...
CHEMISTRY CHAPTER 4 – QUANTUM MECHANICS
... angular momentum quantum number Aufbau principle continuous spectrum electromagnetic radiation electromagnetic spectrum electron configuration excited state frequency ground state Heisenberg uncertainty principle Hund’s rule inner shell electrons line-emission spectrum ...
... angular momentum quantum number Aufbau principle continuous spectrum electromagnetic radiation electromagnetic spectrum electron configuration excited state frequency ground state Heisenberg uncertainty principle Hund’s rule inner shell electrons line-emission spectrum ...
Chapter 9: Chemical Quantities
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
Chapter7_1 - Department of Chemistry [FSU]
... 7.96. Electric power is typically stated in units of watts (1W = 1J/s). About 95% of the power output of an incandescent bulb is converted to heat and 5% to light. If 10% of that light shines on your chemistry text, how many photons per second shine on the book from a 75-W bulb? (assume a wavelenght ...
... 7.96. Electric power is typically stated in units of watts (1W = 1J/s). About 95% of the power output of an incandescent bulb is converted to heat and 5% to light. If 10% of that light shines on your chemistry text, how many photons per second shine on the book from a 75-W bulb? (assume a wavelenght ...
Early Quantum Theory Powerpoint
... an electron from the ground state is called the ionization energy For hydrogen is it 13.6eV and precisely corresponds to the energy to go from E1 to E=0 Often shown in an Energy Level Diagram Vertical arrows show transitions Energy released or absorvedcan be calculated by the difference between ...
... an electron from the ground state is called the ionization energy For hydrogen is it 13.6eV and precisely corresponds to the energy to go from E1 to E=0 Often shown in an Energy Level Diagram Vertical arrows show transitions Energy released or absorvedcan be calculated by the difference between ...
Ch4 notes - Midway ISD
... electrons in same orbital, opposite spin • Hund’s rule – orbitals of equal energy must all have 1 electrons before a second can ...
... electrons in same orbital, opposite spin • Hund’s rule – orbitals of equal energy must all have 1 electrons before a second can ...
The Nature of Light
... rainbow of colors without any spectral line • Law 2 – emission line spectrum: a hot, transparent gas produces an emission line spectrum – a series of bright spectral lines against a dark background • Law 3 – absorption line spectrum: a relatively cool, transparent gas in front of a source of a conti ...
... rainbow of colors without any spectral line • Law 2 – emission line spectrum: a hot, transparent gas produces an emission line spectrum – a series of bright spectral lines against a dark background • Law 3 – absorption line spectrum: a relatively cool, transparent gas in front of a source of a conti ...
Photon momentum and uncertainty
... Transforms optical energy to electrical energy through the use of a photoconductive surface. The idea is similar to that used in the ubiquitous office copier machine. In xerography, a document is scanned and transferred onto a photosensitive drum, which attracts dyes of carbon particles that are rol ...
... Transforms optical energy to electrical energy through the use of a photoconductive surface. The idea is similar to that used in the ubiquitous office copier machine. In xerography, a document is scanned and transferred onto a photosensitive drum, which attracts dyes of carbon particles that are rol ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.
















![Chapter7_1 - Department of Chemistry [FSU]](http://s1.studyres.com/store/data/016128835_1-aea3c1aec04363d6cbf538e8faf80e45-300x300.png)





