Section 5-1
... certain elements emitted visible light when heated in a flame. • Analysis of the emitted light revealed that an element’s chemical behavior is related to the arrangement of the electrons in its atoms. ...
... certain elements emitted visible light when heated in a flame. • Analysis of the emitted light revealed that an element’s chemical behavior is related to the arrangement of the electrons in its atoms. ...
Analytical technique: Fluorescence Spectroscopy
... emission and excitation spectra of materials which may provide indications of the presence (qualitative) of fluorophores or chromophores. When applied to solid samples, the technique is totally non destructive. The analysis of very dilute solutions (<1 mg in a suitable solvent) can lead to the detec ...
... emission and excitation spectra of materials which may provide indications of the presence (qualitative) of fluorophores or chromophores. When applied to solid samples, the technique is totally non destructive. The analysis of very dilute solutions (<1 mg in a suitable solvent) can lead to the detec ...
Preview of Period 3: Electromagnetic Waves – Radiant Energy II
... 3.1: The quantum model describes radiant energy as composed of small packets of energy called photons or quanta. If an electron in the shell of an atom absorbs a photon, the electron is raised to a higher energy level. The electron can emit one or more photons by dropping back to a lower energy leve ...
... 3.1: The quantum model describes radiant energy as composed of small packets of energy called photons or quanta. If an electron in the shell of an atom absorbs a photon, the electron is raised to a higher energy level. The electron can emit one or more photons by dropping back to a lower energy leve ...
Modern Physics Review - hhs
... Radioactivity is the act of atoms breaking apart. They do this because the protons in the nucleus repel each other. Some atoms are more unstable then others because they have too many protons and not the right amount of glue (neutrons) holding them together. We can predict which isotopes are stable ...
... Radioactivity is the act of atoms breaking apart. They do this because the protons in the nucleus repel each other. Some atoms are more unstable then others because they have too many protons and not the right amount of glue (neutrons) holding them together. We can predict which isotopes are stable ...
Chapt7
... Energetically excited atoms only emit radiation in discrete energies corresponding to the atom's electronic energy levels. (see Figure 7.11) ...
... Energetically excited atoms only emit radiation in discrete energies corresponding to the atom's electronic energy levels. (see Figure 7.11) ...
Excited Elements - Light Emission Spectroscopy
... As electrons absorb energy they become excited and move to higher energy levels. As the electrons fall back to lower energy levels they release the energy they absorbed in set amounts called quanta. The energy that is released as electrons fall from higher to lower energy levels has a characteristic ...
... As electrons absorb energy they become excited and move to higher energy levels. As the electrons fall back to lower energy levels they release the energy they absorbed in set amounts called quanta. The energy that is released as electrons fall from higher to lower energy levels has a characteristic ...
No Slide Title - Rubin Gulaboski
... Line Spectra Radiation composed of only one wavelength is called monochromatic. Most common radiation sources that produce radiation containing many different wavelengths components, a spectrum. This rainbow of colors, containing light of all wavelengths, is called a continuous spectrum. Note that t ...
... Line Spectra Radiation composed of only one wavelength is called monochromatic. Most common radiation sources that produce radiation containing many different wavelengths components, a spectrum. This rainbow of colors, containing light of all wavelengths, is called a continuous spectrum. Note that t ...
Atomic Physics 4
... and eventually collapse into the nucleus. – Obviously, this does not happen. ...
... and eventually collapse into the nucleus. – Obviously, this does not happen. ...
Electronic Structure of Atoms
... • Line spectra result from the emission of radiation from an excited atom. • Spectrum: characteristic pattern of wavelengths absorbed (or emitted) by a substance. • Emission Spectrum: spontaneous emission of radiation from an excited atom or molecule. • Line Spectrum: spectrum containing only certai ...
... • Line spectra result from the emission of radiation from an excited atom. • Spectrum: characteristic pattern of wavelengths absorbed (or emitted) by a substance. • Emission Spectrum: spontaneous emission of radiation from an excited atom or molecule. • Line Spectrum: spectrum containing only certai ...
Fourier Transform Infrared (FTIR) Spectroscopy
... This light wave has many frequencies. And the frequency increases in time (from red to blue). Spectra can be measured using dispersive instruments, or an interferometer ...
... This light wave has many frequencies. And the frequency increases in time (from red to blue). Spectra can be measured using dispersive instruments, or an interferometer ...
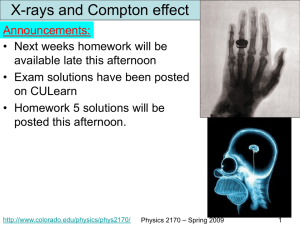
Physics 2170
... A. Destructive interference occurs when the path length difference is (n+½)l. B. For a given m≠0, blue light gets scattered at a larger angle than red light. C. A green laser pointer with l = 532 nm will show a rainbow of colors when sent through an appropriate diffraction grating. D. More than one ...
... A. Destructive interference occurs when the path length difference is (n+½)l. B. For a given m≠0, blue light gets scattered at a larger angle than red light. C. A green laser pointer with l = 532 nm will show a rainbow of colors when sent through an appropriate diffraction grating. D. More than one ...
W11Physics1CLec28Afkw
... But if the energy of the photon is greater than the work function of the metal then the electron will be liberated and given kinetic energy, as well. The maximum kinetic energy of the liberated photoelectron is: ...
... But if the energy of the photon is greater than the work function of the metal then the electron will be liberated and given kinetic energy, as well. The maximum kinetic energy of the liberated photoelectron is: ...
Photoelectric Effect Practice Problems
... 12. In studying a solid material for possible use in a solar cell (which turns light into electrical energy), material engineers shine a monochromatic blue light (λ = 420 nm) to produce photoelectrons. They measure the maximum kinetic energy of the emitted electrons to be 1.00 x 10-19 J. Predict wha ...
... 12. In studying a solid material for possible use in a solar cell (which turns light into electrical energy), material engineers shine a monochromatic blue light (λ = 420 nm) to produce photoelectrons. They measure the maximum kinetic energy of the emitted electrons to be 1.00 x 10-19 J. Predict wha ...
Photoelectric Effect Practice Problems
... 12. In studying a solid material for possible use in a solar cell (which turns light into electrical energy), material engineers shine a monochromatic blue light (λ = 420 nm) to produce photoelectrons. They measure the maximum kinetic energy of the emitted electrons to be 1.00 x 10-19 J. Predict wha ...
... 12. In studying a solid material for possible use in a solar cell (which turns light into electrical energy), material engineers shine a monochromatic blue light (λ = 420 nm) to produce photoelectrons. They measure the maximum kinetic energy of the emitted electrons to be 1.00 x 10-19 J. Predict wha ...
UV and IR Spectra to Determine Simulated Astrophysical Species
... Lyman-α photons (10.2 eV), 5-keV electrons, 60keV Ar2+ ions and so on. From the existence of CH4 in N2 ice, products such as CH3, C2H2, HNC, HCN, C2H6, CH2N2, and HCN2 were identified. Pure N2 ice and of solid N2 with dilute CH4 have been subjected to photolysis at various wavelengths using synchrot ...
... Lyman-α photons (10.2 eV), 5-keV electrons, 60keV Ar2+ ions and so on. From the existence of CH4 in N2 ice, products such as CH3, C2H2, HNC, HCN, C2H6, CH2N2, and HCN2 were identified. Pure N2 ice and of solid N2 with dilute CH4 have been subjected to photolysis at various wavelengths using synchrot ...
Resonant X-ray Emission Spectroscopy
... shallow core-hole in the final state (τ ~ several fs) compared with the intermediate core hole state (τ ~ fs). Since the width of the measured RXES spectrum depends only on the final state lifetime broadening this kind of RXES measurements can be applied to record absorption spectrum free from lifet ...
... shallow core-hole in the final state (τ ~ several fs) compared with the intermediate core hole state (τ ~ fs). Since the width of the measured RXES spectrum depends only on the final state lifetime broadening this kind of RXES measurements can be applied to record absorption spectrum free from lifet ...
Slide 1
... Periodic Table have similar chemical properties. This similarity is most closely related to the atoms‘ 1. number of principal energy levels 2. number of valence electrons 3. atomic numbers 4. atomic masses ...
... Periodic Table have similar chemical properties. This similarity is most closely related to the atoms‘ 1. number of principal energy levels 2. number of valence electrons 3. atomic numbers 4. atomic masses ...
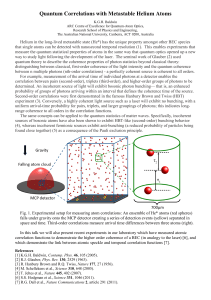
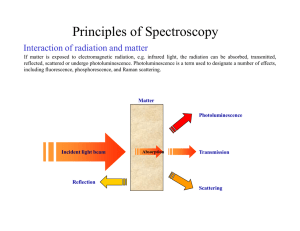
Principles of Spectroscopy
... Origin of the interferogram Spectrometers are equipped with a broadband light source, which yields a continuous, infinite number, of wavelengths, as shown in the figure on the left. The interferogram is the continuous sum, i.e. the integral, of all the interference patterns produced by each wavelen ...
... Origin of the interferogram Spectrometers are equipped with a broadband light source, which yields a continuous, infinite number, of wavelengths, as shown in the figure on the left. The interferogram is the continuous sum, i.e. the integral, of all the interference patterns produced by each wavelen ...
Slide 1 - KaiserScience
... wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. ...
... wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. ...
SignalsInstr
... telling you something about the physics of the sample. However, it could also be telling you about the physics of the instrumentation. For example, the light source might not have the same intensity at all wavelengths, the mirrors or gratings in the monochromator might have different efficiencies at ...
... telling you something about the physics of the sample. However, it could also be telling you about the physics of the instrumentation. For example, the light source might not have the same intensity at all wavelengths, the mirrors or gratings in the monochromator might have different efficiencies at ...
PPA6_Lecture_Ch_27
... wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. ...
... wave would be a circular standing wave will occur. This yields the same relation that Bohr had proposed. ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.