Document
... Review Problem: Suppose that the electron in the hydrogen atom obeyed classical rather then quantum mechanics. Why should such an atom emit a continuous rather then discrete spectrum? If hydrogen obeyed classical physics, we would have no quantized electron orbits. Therefore the transitions between ...
... Review Problem: Suppose that the electron in the hydrogen atom obeyed classical rather then quantum mechanics. Why should such an atom emit a continuous rather then discrete spectrum? If hydrogen obeyed classical physics, we would have no quantized electron orbits. Therefore the transitions between ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... is one fourth its rest energy, what is its speed? 12) Establish the invariance of E.B under Lorentz transformation. 13) Outline the Green’s function method of obtaining obtaining a formal solution of the Schroedinger wave equation in scattering theory. 14) Discuss the time-dependent dependent pertur ...
... is one fourth its rest energy, what is its speed? 12) Establish the invariance of E.B under Lorentz transformation. 13) Outline the Green’s function method of obtaining obtaining a formal solution of the Schroedinger wave equation in scattering theory. 14) Discuss the time-dependent dependent pertur ...
AP Semester I Review: Free Response Questions
... The structures of a water molecule and a crystal of LiCl(s) are represented above. A student prepares a 1.0 M solution by dissolving 4.2 g of LiCl(s) in enough water to make 100 mL of solution. a. In the space provided below, show the interactions of the components of LiCl(aq) by making a drawing th ...
... The structures of a water molecule and a crystal of LiCl(s) are represented above. A student prepares a 1.0 M solution by dissolving 4.2 g of LiCl(s) in enough water to make 100 mL of solution. a. In the space provided below, show the interactions of the components of LiCl(aq) by making a drawing th ...
Unit 2 Review KEY
... Electromagnetic Radiation – form of energy that exhibits wavelength behavior as it travels through space. Wavelength (λ) – the distance between corresponding points on adjacent waves. Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emissio ...
... Electromagnetic Radiation – form of energy that exhibits wavelength behavior as it travels through space. Wavelength (λ) – the distance between corresponding points on adjacent waves. Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emissio ...
ppt - University Of Oregon
... Built within constraints: Resonance no higher than 13MHz. See CW ...
... Built within constraints: Resonance no higher than 13MHz. See CW ...
Electromagnetic Waves and Photons are describing the same thing
... 1. What one sees if bash atoms with anything, particularly electrons, as in a discharge lamp. 2. What light coming from atoms (“spectra”) imply about behavior of electrons in atom. ...
... 1. What one sees if bash atoms with anything, particularly electrons, as in a discharge lamp. 2. What light coming from atoms (“spectra”) imply about behavior of electrons in atom. ...
Lecture 13: Heisenberg and Uncertainty
... Transforms optical energy to electrical energy through the use of a photoconductive surface. The idea is similar to that used in the ubiquitous office copier machine. In xerography, a document is scanned and transferred onto a photosensitive drum, which attracts dyes of carbon particles that are rol ...
... Transforms optical energy to electrical energy through the use of a photoconductive surface. The idea is similar to that used in the ubiquitous office copier machine. In xerography, a document is scanned and transferred onto a photosensitive drum, which attracts dyes of carbon particles that are rol ...
WBL6_Lecture_Ch27
... Thermal radiation depends only on the temperature of the radiating body. The peak wavelength increases with temperature. Classical theory could not predict the thermal radiation spectrum; Planck did so by assuming that the energies of the atoms in the material were quantized. ...
... Thermal radiation depends only on the temperature of the radiating body. The peak wavelength increases with temperature. Classical theory could not predict the thermal radiation spectrum; Planck did so by assuming that the energies of the atoms in the material were quantized. ...
Louie de Broglie
... describe the properties, such as the energy level and shape (s, p, d or f), and Orientation of the atomic orbitals. ...
... describe the properties, such as the energy level and shape (s, p, d or f), and Orientation of the atomic orbitals. ...
Chapter 7: Electrons in Atoms Electromagnetic Radiation
... As nf goes to infinity for hydrogen starting in the ground state: hν = RH ( ...
... As nf goes to infinity for hydrogen starting in the ground state: hν = RH ( ...
Chapter 5 Review “Electrons in Atoms”
... or b) wavelength? Which color of visible light has the shortest wavelength? The quantum mechanical model of the atom involves the _____. ...
... or b) wavelength? Which color of visible light has the shortest wavelength? The quantum mechanical model of the atom involves the _____. ...
Chapter 5 Review “Electrons in Atoms”
... or b) wavelength? Which color of visible light has the shortest wavelength? The quantum mechanical model of the atom involves the _____. ...
... or b) wavelength? Which color of visible light has the shortest wavelength? The quantum mechanical model of the atom involves the _____. ...
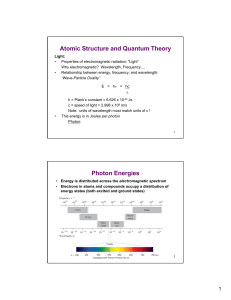
Atomic Structure and Quantum Theory Photon Energies
... • Based on wave/particle behavior of electrons and electromagnetic radiation (light) Seeks to answer questions like: • Why do different atoms emit different colors? • Why are molecules shaped as they are? Wave-particle duality causes a problem. If we consider electrons to have wave properties, how c ...
... • Based on wave/particle behavior of electrons and electromagnetic radiation (light) Seeks to answer questions like: • Why do different atoms emit different colors? • Why are molecules shaped as they are? Wave-particle duality causes a problem. If we consider electrons to have wave properties, how c ...
Lab Science 9 Pacing Guide
... 4. Show that when elements are listed in order according to the number of protons (called the atomic number), the repeating patterns of physical and chemical properties identify families of elements. Recognize that the periodic table was formed as a result of the repeating pattern of electron config ...
... 4. Show that when elements are listed in order according to the number of protons (called the atomic number), the repeating patterns of physical and chemical properties identify families of elements. Recognize that the periodic table was formed as a result of the repeating pattern of electron config ...
laser beam smoothing by optical fiber for improvement of the target
... After the fiber, laser radiation passes through the preamplifiers, the systems of temporal and spatial shaping and is fed in the main amplifier channel of the Luch facility. Amplified radiation at the channel’s exit converts into second harmonic and is focused on the target. The radiation spot shape ...
... After the fiber, laser radiation passes through the preamplifiers, the systems of temporal and spatial shaping and is fed in the main amplifier channel of the Luch facility. Amplified radiation at the channel’s exit converts into second harmonic and is focused on the target. The radiation spot shape ...
Chapter 7- Components of Optical Instruments
... level Ev""- The lifetime of excited vibrational states is brief, and after 10-13 to 10-15 S relaxation to the lowest excited vibrational level. Some excited electronic states of laser materials have lifetimes considerably longer (often 1 ms or more) than their vibration counterparts; long-lived stat ...
... level Ev""- The lifetime of excited vibrational states is brief, and after 10-13 to 10-15 S relaxation to the lowest excited vibrational level. Some excited electronic states of laser materials have lifetimes considerably longer (often 1 ms or more) than their vibration counterparts; long-lived stat ...
Problem-set-6
... A “quantum defect” indicates in how far the level energies in a certain atom deviate from the energy of “hydrogenic orbitals”, i.e. the orbitals in an hydrogen atom. Such information can be derived from the spectra of atoms. From combinations of spectral lines the level energies in an atom can then ...
... A “quantum defect” indicates in how far the level energies in a certain atom deviate from the energy of “hydrogenic orbitals”, i.e. the orbitals in an hydrogen atom. Such information can be derived from the spectra of atoms. From combinations of spectral lines the level energies in an atom can then ...
Bio321_2010 slides
... used as contrast agents. • Contrast (and resolution) enhancement can be obtained by two-photon excitation and ...
... used as contrast agents. • Contrast (and resolution) enhancement can be obtained by two-photon excitation and ...
Document
... Cutoff frequency Photoelectrons are created by absorption of a single photon that has enough energy to overcome the work function. Independence of KEmax of light intensity KEmax depends on only the frequency of light and the work function. Linear dependence of KEmax on light frequency ...
... Cutoff frequency Photoelectrons are created by absorption of a single photon that has enough energy to overcome the work function. Independence of KEmax of light intensity KEmax depends on only the frequency of light and the work function. Linear dependence of KEmax on light frequency ...
Laboratory 3: Determining the Critical Potentials for Helium: The
... the states of the atom quantized. He predicted that the electrons in atoms can only exist in certain bound states (energy levels). In 1914, J. Franck and G. Hertz confirmed the Bohr model for atoms that electrons only occupy discrete quantized energy levels and made the first non-optical measurement ...
... the states of the atom quantized. He predicted that the electrons in atoms can only exist in certain bound states (energy levels). In 1914, J. Franck and G. Hertz confirmed the Bohr model for atoms that electrons only occupy discrete quantized energy levels and made the first non-optical measurement ...
Atomic Radius and Ionization Energy
... Trends in Ionization Energy • Energy needed to remove an electron from an atom • Energy needed to remove the first electron from an atom is the FIRST ...
... Trends in Ionization Energy • Energy needed to remove an electron from an atom • Energy needed to remove the first electron from an atom is the FIRST ...
Chapter 5 Practice Section 5-1 Discuss the placement (if any) of
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
... What is the wavelength of radiation with a frequency of 2.3 x 1014 Hz? What is the frequency of radiation with a wavelength of 1.8 x 10-9 m? Rank in order of increasing energy: Purple light, x-rays, Microwaves Rank the above in order of increasing frequency. Rank the above in order of increasing wav ...
The Quantum Atom (section 18)
... Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
... Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
Section 5.3 Physics and Quantum Mechanical Model
... element passes through a prism, it separates into discrete lines to give the atomic emission spectrum of that element. ...
... element passes through a prism, it separates into discrete lines to give the atomic emission spectrum of that element. ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.