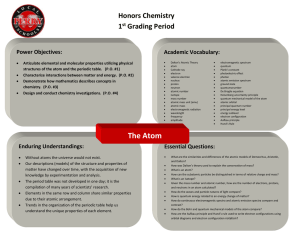
Hydrogen Atom Simulator – Exercises
... Increase the energy more until photons of range 3 are reached. In the simulator this will be just above the Lε line. o Technically there are photons which would excite to the 7th, 8th, 9th, etc. energy levels, but these are very close together and those lines not shown on the simulator. o The Lε lin ...
... Increase the energy more until photons of range 3 are reached. In the simulator this will be just above the Lε line. o Technically there are photons which would excite to the 7th, 8th, 9th, etc. energy levels, but these are very close together and those lines not shown on the simulator. o The Lε lin ...
1 Lecture 19 Physics 404 We considered the free electrons in a
... lecture that their total energy would be by equipartition of energy. Their heat capacity would be , which is substantial and temperature independent. However the electronic heat capacity of metals is observed to be much smaller than this and linear in temperature. The discrepancy is explained by the ...
... lecture that their total energy would be by equipartition of energy. Their heat capacity would be , which is substantial and temperature independent. However the electronic heat capacity of metals is observed to be much smaller than this and linear in temperature. The discrepancy is explained by the ...
Shiny, Happy Pretest - Alex LeMay – Science
... that Rutherford should let Marsden get some lab experience. __________________________ 15. Believed that the world was made of matter that could be divided infinitely. _____________ 16. Figured out that radiation can be divided into alpha particles, beta particles and gamma rays and that atoms were ...
... that Rutherford should let Marsden get some lab experience. __________________________ 15. Believed that the world was made of matter that could be divided infinitely. _____________ 16. Figured out that radiation can be divided into alpha particles, beta particles and gamma rays and that atoms were ...
energy
... emit or absorb energy only in wholenumber multiples of hn; that is, 1hn, 2hn, 3hn, and so on. • Matter can have only certain amounts of energy—quantities of energy between these values do not exist. ...
... emit or absorb energy only in wholenumber multiples of hn; that is, 1hn, 2hn, 3hn, and so on. • Matter can have only certain amounts of energy—quantities of energy between these values do not exist. ...
PHYS 221: Homework Assignment 3 This homework due just prior
... a) [6 points] What is the greatest possible value that the wavelength λ of the electron could have and still be consistent with these facts? [Give your answer in terms of the given fixed quantities a and θ] b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelengt ...
... a) [6 points] What is the greatest possible value that the wavelength λ of the electron could have and still be consistent with these facts? [Give your answer in terms of the given fixed quantities a and θ] b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelengt ...
Electron Configuration
... ◦ Orbitals are sometimes called electron clouds because they do not have sharp boundaries ◦ Tells where it is likely to find an electron ...
... ◦ Orbitals are sometimes called electron clouds because they do not have sharp boundaries ◦ Tells where it is likely to find an electron ...
Topic 2 IB Chemistry Assessment Statements 2009 Revised File
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
Stimulated Emission of Radiation
... statistics start to play a role - for lighter elements with smaller masses the oscillator frequencies are higher and thus quantum effects are more important even at higher temperatures - the zero point motion does not play a role for the specific heat as it is only a constant offset in energy ...
... statistics start to play a role - for lighter elements with smaller masses the oscillator frequencies are higher and thus quantum effects are more important even at higher temperatures - the zero point motion does not play a role for the specific heat as it is only a constant offset in energy ...
kinetic energy of photoelectrons (eV)
... • The energy of a photon is very small, a more convenient unit is the “electron volt” (eV) the amount of energy an electron gains or loses as it moves through a potential difference of 1 volt ...
... • The energy of a photon is very small, a more convenient unit is the “electron volt” (eV) the amount of energy an electron gains or loses as it moves through a potential difference of 1 volt ...
Quantum Theory of Light. Matter Waves.
... aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electrons also show evidence of behaving as waves (diffraction, interference). The wave-partic ...
... aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electrons also show evidence of behaving as waves (diffraction, interference). The wave-partic ...
Periodic Trends
... First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
... First ionization energy increases from left to right across a period. First ionization energy decreases down a group because atomic size increases and less energy is required to remove an electron farther from the nucleus. ...
explo3
... Where, En are the energy eigen values without any corrections. 2) The above equation gives the energy levels with fine structure corrections. a) Show that the correction term does not vanish for any possible combination of n and j, but always reduces the value of uncorrected energy. b) In how many e ...
... Where, En are the energy eigen values without any corrections. 2) The above equation gives the energy levels with fine structure corrections. a) Show that the correction term does not vanish for any possible combination of n and j, but always reduces the value of uncorrected energy. b) In how many e ...
Chapter 2 - Speedway High School
... • Atoms of the various elements differ in number of subatomic particles – Atomic number – Mass number – Atomic mass ...
... • Atoms of the various elements differ in number of subatomic particles – Atomic number – Mass number – Atomic mass ...
What is Light?
... • Combined planetary model and quantum mechanics • Electrons orbit nucleus, but only certain orbits allowed • Angular momentum quantized: L = (mr)v = n(h/2π) n=1,2,3… • Fit mathematical prediction of H spectra by Balmer (1885) • Electrons in atoms cannot lose/gain energy continuously (Planck/Einstei ...
... • Combined planetary model and quantum mechanics • Electrons orbit nucleus, but only certain orbits allowed • Angular momentum quantized: L = (mr)v = n(h/2π) n=1,2,3… • Fit mathematical prediction of H spectra by Balmer (1885) • Electrons in atoms cannot lose/gain energy continuously (Planck/Einstei ...
Chemistry: The Nature of Matter
... o 2nd shell has a little more energy and holds 8 electrons o 3rd shell has even more energy, etc. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Electron config ...
... o 2nd shell has a little more energy and holds 8 electrons o 3rd shell has even more energy, etc. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ Electron config ...
View the Powerpoint Presentation.
... Discovered for the first time by Max Planck and Heinrich Hertz, Germany, in the late 1800’s. ...
... Discovered for the first time by Max Planck and Heinrich Hertz, Germany, in the late 1800’s. ...
1. Crystal Properties and Growth of Semiconductors
... Shortest Course in Quantum Mechanics, Cont. Heisenberg Uncertainty Principle (Nobel prize 1932 for the creation of quantum mechanics) The more precise you know the position of a particle , the less precise you know the momentum of the ...
... Shortest Course in Quantum Mechanics, Cont. Heisenberg Uncertainty Principle (Nobel prize 1932 for the creation of quantum mechanics) The more precise you know the position of a particle , the less precise you know the momentum of the ...
QUANTUM THEORY
... 16. Which one of the following statements concerning photons is false? A) Photons have zero mass. B) The rest energy of all photons is zero. C) Photons travel at the speed of light in a vacuum. D) Photons have been brought to rest by applying a strong magnetic field to them. E) The energy of a photo ...
... 16. Which one of the following statements concerning photons is false? A) Photons have zero mass. B) The rest energy of all photons is zero. C) Photons travel at the speed of light in a vacuum. D) Photons have been brought to rest by applying a strong magnetic field to them. E) The energy of a photo ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.