* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture24 - Purdue Physics
Night vision device wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Photoacoustic effect wikipedia , lookup
Atmospheric optics wikipedia , lookup
Image intensifier wikipedia , lookup
Retroreflector wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Nonlinear optics wikipedia , lookup
Photomultiplier wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Opto-isolator wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Transparency and translucency wikipedia , lookup
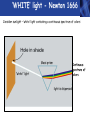
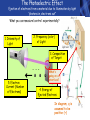
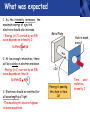
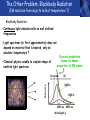
PHYS219 Fall semester 2014 Lecture 24: Quantum Optics: Photons (moving to Chapter 28) Dimitrios Giannios Purdue University Continuous and Quantized For the remainder of the course, it will become increasingly important to distinguish between continuous and quantized items. Some things seem inherently continuous (like length, energy, mass) while other entities seem inherently discrete or quantized (like money, number of people at a football game, etc.) In science, the existence of atoms (different chemical elements) provided first hint that nature is quantized. • Light is especially interesting because it displays different behavior in different situations • Ray optics – lenses & mirrors • Wave optics – interference & diffraction • Quantum optics – interaction w. electrons continuous quantized Visible EM Radiation • from Maxwell in the 1860s, we know that light is an EM wave. • in 1888, Hertz showed that EM waves with long wavelengths could be launched and detected using transmitters and receivers (the forerunners of today’s communication industry). • …………but what about visible light? How is it generated? Consider a few sources of visible light: • Sun • Fire • Oil lamp • Gas discharge tube • Heat radiation (blackbody) ‘WHITE’ light - Newton 1666 Consider sunlight – ‘white’ light containing a continuous spectrum of colors Glass prism Continuous spectrum of colors “white” light light is dispersed Newton’s Result–inconsistency with light from Gas Discharge Tube - Discrete Wavelengths (late 1800s) Glass tube, evacuated and back-filled with gas Glass prism light is dispersed from Gas Discharge Lamp Use diffraction grating to accurately measure wavelengths bright fringes (bright spots) incident light λ1, λ2, λ3, etc multiple slits separation between slits = d Note: Each λ produces a different y y W >> y Viewing screen Spectral Lines for Common Gases Wavelengths accurately measured Why are there so many lines? Check out http://www.colorado.edu/physics/2000/applets/a2.html Focus on light emitted from Hydrogen discharge tube Balmer’s empirical formula (1884) explains the observed visible wavelengths from hydrogen gas with high accuracy n=7 n=6 n=5 n=4 é1 1ù = R H ê - ú ; n = 3, 4,5... êë22 n 2 úû l 1 RH = 1.097 × 107 m-1 n=3 The Photoelectric Effect Ejection of electrons from a material due to illumination by light “photons in, electrons out” What you can measure/control experimentally? 1. Intensity of Light 2. Frequency (color) of Light 3. Composition of Target e- 5. Electron Current (Number of Electrons) e- e- What’s going on at interface? 4. Energy of Ejected Electrons In diagram, q is assumed to be positive (+) 9 Check out the photoelectric simulation http://phet.colorado.edu/new/simulations/sims.php?sim=Photoelectric_Effect What was expected 1. As the intensity increases, the maximum energy of ejected electrons should also increase. - Energy (in J) carried by an EM wave depends on intensity I: Metal Plate U=PΔt=(I⨯A)Δt 2. At low enough intensities, there will be a delay in electron emission. - Energy (in J) carried by an EM wave depends on time Δt: U=PΔt=(I ⨯ A)Δt 3. Electrons should be emitted for all wavelengths of light. -The wavelength λ does not appear in above equations Hole in mask, area A utot cΔt Energy U passing thru hole in time Δt Time aver. radiation, intensity I What was observed a) Red light x2 x2 4Io 2Io Io No electron emission - ever! Metal plate b) Blue light Io Metal plate x2 x2 Prompt electron emission even at lowest intensity! 2Io 4Io Why the Photoelectric Effect was so Surprising a) Low frequency water waves Large amplitude, any λ ? h b) High frequency water waves ? High frequency, small λ h When does the fisherman leave the dock? The Other Problem: Blackbody Radiation (EM radiation from objects held at temperature T) Blackbody Radiation: • Continuous light emission with no well defined frequencies • Light spectrum (to first approximation) does not depend on material that is heated, only on absolute temperature T • Classical physics unable to explain shape of emitted light spectrum Classical prediction based on known properties of EM waves Intensity UV visible IR 6000 K 3000 K 1000 nm 2000 nm Wavelength,λ Heat (blackbody) Radiation See: http://phet.colorado.edu/simulations/sims.php?sim=Blackbody_Spectrum Planck’s Hypothesis (1900) To quantitatively explain blackbody radiation spectra, Planck assumes that a blackbody is made of “atomic oscillators” that emit light which is quantized in energy, ie E = n ⨯(hf) h= 6.626 x 10-34 J s (Planck’s Constant) n = integer f= frequency of light This simple idea had an impact similar to the impact of Newton’s equations in the 1750’s Einstein (in 1905) hypothesized Planck’s idea (in 1900) followed up by Einstein (in 1905) hypothesized that light itself must be thought of as quantized packets of energy called photons one photon Ephoton = hf n=6 Light beam n=9 n=3 Foundation of QUANTUM optics Why is this quantization hypothesis so surprising? Since light is a wave (only way to explain interference and diffraction effects from the early 1800’s), everyone thought that it’s energy could be varied continuously by adjusting the wave’s amplitude. This simple idea was completely contradicted by Planck’s hypothesis.