File
... famous uncertainty principle (better translated, however, as “indeterminacy principle”) states that the more accurately an object’s position can be observed, the less accurately its momentum can. This is because shorter wavelengths of light (use as a sort of measuring-stick) have higher energies, a ...
... famous uncertainty principle (better translated, however, as “indeterminacy principle”) states that the more accurately an object’s position can be observed, the less accurately its momentum can. This is because shorter wavelengths of light (use as a sort of measuring-stick) have higher energies, a ...
1.1 What has to be explained by Quantum mechanics?
... • What is: Schrödinger equation, Operator, commutator, probability function, wave function, quantum number, ...... ...
... • What is: Schrödinger equation, Operator, commutator, probability function, wave function, quantum number, ...... ...
Ch 6 Outline
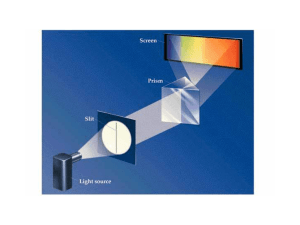
... Photoelectric effect Emission spectra Quantum Calculate the energy of one photon of yellow light with a wavelength of 589nm. ...
... Photoelectric effect Emission spectra Quantum Calculate the energy of one photon of yellow light with a wavelength of 589nm. ...
Quantum Mechanical Energy and You!
... In the new realm of thought, macroscopic objects now be thought of in terms of their matter waves or Debroglie waves. Waves are quantities that are highly non local and spread through space in time. Thus, the idea of how we talk of the fundamental quantity position must be re-invented, as well as al ...
... In the new realm of thought, macroscopic objects now be thought of in terms of their matter waves or Debroglie waves. Waves are quantities that are highly non local and spread through space in time. Thus, the idea of how we talk of the fundamental quantity position must be re-invented, as well as al ...
Atomic Structure
... Introduction to waves: You are familiar with many types of radiant energy: visible light, UV-light, X-rays, and gamma radiation. James Clerk Maxwell in 1856 proposed the existence of waves which were related to both electricity and magnetism and called them Electromagnetic waves. Some waves require ...
... Introduction to waves: You are familiar with many types of radiant energy: visible light, UV-light, X-rays, and gamma radiation. James Clerk Maxwell in 1856 proposed the existence of waves which were related to both electricity and magnetism and called them Electromagnetic waves. Some waves require ...
Chapter 4-Arrangement of Electrons in Atoms
... c = speed of light (3.00 x 10 m/sec) = wavelength a. What is the wavelength of a radiation whose frequency is 1.50 x 10 ...
... c = speed of light (3.00 x 10 m/sec) = wavelength a. What is the wavelength of a radiation whose frequency is 1.50 x 10 ...
stringtheory1s
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
Particle-like Properties of Electromagnetic Radiation
... that as the object was heat at a higher temperature, the colour (or the radiant light) of the object changed from long (low energy) to shorter (higher energy). But this rising trend did not continue indefinitely. Planck continued that energy radiated by a heated object is constrained to discrete amo ...
... that as the object was heat at a higher temperature, the colour (or the radiant light) of the object changed from long (low energy) to shorter (higher energy). But this rising trend did not continue indefinitely. Planck continued that energy radiated by a heated object is constrained to discrete amo ...
Developing a new physics for atoms
... In 1927, Werner Heisenberg found that pairs of properties of particles cannot have exact values at the same time when the particles are of subatomic scale. He called this ‘indeterminant’ behavior. For example, if you know a subatomic particle’s speed or momentum exactly, you cannot know its location ...
... In 1927, Werner Heisenberg found that pairs of properties of particles cannot have exact values at the same time when the particles are of subatomic scale. He called this ‘indeterminant’ behavior. For example, if you know a subatomic particle’s speed or momentum exactly, you cannot know its location ...
Announcements
... packages called photons (particles) l Electrons that move through space in straight lines and experience collisions as if they were particles distribute themselves in interference patterns as if they were waves l Light and electrons exhibit both wave and particle characteristics l Niels Bohr c ...
... packages called photons (particles) l Electrons that move through space in straight lines and experience collisions as if they were particles distribute themselves in interference patterns as if they were waves l Light and electrons exhibit both wave and particle characteristics l Niels Bohr c ...
Quantum Mechanics Lecture Course for 4 Semester Students by W.B. von Schlippe
... particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles, such as electrons or protons, have properties characteristic of both waves ...
... particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles, such as electrons or protons, have properties characteristic of both waves ...
Liad Elmelech 7.1-7.3 The Nature of Light, Atomic Spectroscopy
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
... binding energy(φ) • hv = φ • Low frequency light does not eject electrons because no single photon has enough energy to dislodge • Energy of a photon that is beyond what is needed to dislodge an electron is transferred to the electron in the form of kinetic energy • KE = hv – φ ...
Physical Chemistry II Review Set 1
... d. The integral of the wave function over "all space" = 1. 8. For a particle in a box of length 1nm: a. Sketch the ground state. b. Sketch the 3rd excited state. c. Using the principals of calculus, state qualitatively what you know about the derivative of the probability density where it is maximum ...
... d. The integral of the wave function over "all space" = 1. 8. For a particle in a box of length 1nm: a. Sketch the ground state. b. Sketch the 3rd excited state. c. Using the principals of calculus, state qualitatively what you know about the derivative of the probability density where it is maximum ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
... 16. State and prove Ehernfest’s theorem 17. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 18. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 19. What are symmetric and antisymmetric wave functions? Show ...
ph 2811 / 2808 - quantum mechanics
... 6. State and prove Ehernfest’s theorem 7. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 8. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 9. What are symmetric and antisymmetric wave functions? Show that ...
... 6. State and prove Ehernfest’s theorem 7. Solve the Schrodinger equation for a linear harmonic oscillator. Sketch the first two eigenfunctions of the system. 8. Determine the eigenvalue spectrum of angular momentum operators Jz and Jz 9. What are symmetric and antisymmetric wave functions? Show that ...
Final “Intro Quantum Mechanics”
... (a) (T) One needs quantum mechanics to explain the spectrum of blackbody radiation, as classical physics gives the wrong answer. This was the effect that prompted Planck to introduce his constant. (b) (T) One needs quantum mechanics to explain the structure of atoms, as classical physics gives the w ...
... (a) (T) One needs quantum mechanics to explain the spectrum of blackbody radiation, as classical physics gives the wrong answer. This was the effect that prompted Planck to introduce his constant. (b) (T) One needs quantum mechanics to explain the structure of atoms, as classical physics gives the w ...
Quantum mechanics of light dispersion: does the photon have mass?
... most fundamental objections against the atomistic theory of matter proposed by Democritus and his followers in ancient Greece and later espoused by Newton in his corpuscular model. The quantum mechanical concept of wave-particle duality, by contrast, holds that matter behaves as particles in some e ...
... most fundamental objections against the atomistic theory of matter proposed by Democritus and his followers in ancient Greece and later espoused by Newton in his corpuscular model. The quantum mechanical concept of wave-particle duality, by contrast, holds that matter behaves as particles in some e ...
CHM 441: QUANTUM CHEMISTRY
... surrounding it, but this could not be understood using classical mechanics which predicted that the electrons would radiates energy and fall into the ...
... surrounding it, but this could not be understood using classical mechanics which predicted that the electrons would radiates energy and fall into the ...
PHY215: Study Guide for Introductory Quantum Mechanics Explain 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays.
... 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays. 2. The photoelectric effect, Compton Scattering, Planck’s constant: explain how light behaves as though it is made of particles. 3. The de Broglie wavelength, the Davisson‐Germer experiment: explain how electrons (an ...
... 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays. 2. The photoelectric effect, Compton Scattering, Planck’s constant: explain how light behaves as though it is made of particles. 3. The de Broglie wavelength, the Davisson‐Germer experiment: explain how electrons (an ...