photon particle - wave duality
... given by Planck based on a picture in which electromagnetic radiation is carried in particle-like bunches called “photons.” 2. The failure of classical physics to adequately describe the photoelectric effect, and the subsequent successful treatment given by Einstein based on the photon picture. ...
... given by Planck based on a picture in which electromagnetic radiation is carried in particle-like bunches called “photons.” 2. The failure of classical physics to adequately describe the photoelectric effect, and the subsequent successful treatment given by Einstein based on the photon picture. ...
CHEM 347 Quantum Chemistry
... de Broglie extended and quantified the wave particle duality of light to all particles (electrons, protons, baseballs). Using special relativity, Einstein derived an expression for the momentum of a photon (even though it has no mass). Using a similar line of reasoning, de Broglie argued that the wa ...
... de Broglie extended and quantified the wave particle duality of light to all particles (electrons, protons, baseballs). Using special relativity, Einstein derived an expression for the momentum of a photon (even though it has no mass). Using a similar line of reasoning, de Broglie argued that the wa ...
The Wave Nature of Matter - Waterford Public Schools
... speed, v, other than the speed of light will have a wave nature consistent with a wavelength given by the equation: h λ= mν λ ...
... speed, v, other than the speed of light will have a wave nature consistent with a wavelength given by the equation: h λ= mν λ ...
wave-particle duality
... Classical picture: oscillating electromagnetic field causes oscillations in positions of charged particles, which re-radiate in all directions at same frequency and wavelength as ...
... Classical picture: oscillating electromagnetic field causes oscillations in positions of charged particles, which re-radiate in all directions at same frequency and wavelength as ...
WAVE-PARTICLE DUALITY
... Classical picture: oscillating electromagnetic field causes oscillations in positions of charged particles, which re-radiate in all directions at same frequency and wavelength as ...
... Classical picture: oscillating electromagnetic field causes oscillations in positions of charged particles, which re-radiate in all directions at same frequency and wavelength as ...
Chapter 7 Worksheet November 1
... C. The light waves bend around an object D. The light waves disperse into their component colors 8. What might the problem be if our retina could detect low frequency electromagnetic radiation? ...
... C. The light waves bend around an object D. The light waves disperse into their component colors 8. What might the problem be if our retina could detect low frequency electromagnetic radiation? ...
l - Gordon State College
... What is the energy of one photon from a yellow light whose wavelength is 589 nm? 5.09 x 1014 Hz ...
... What is the energy of one photon from a yellow light whose wavelength is 589 nm? 5.09 x 1014 Hz ...
Max Planck suggested that the energy of light is proportional to its
... prism can be used to separate the wavelengths, making them easy to identify. If light acted only as a wave, then there should be a continuous rainbow created by the prism. Instead, there are discrete lines created by different wavelengths. This is because electrons release specific wavelengths of li ...
... prism can be used to separate the wavelengths, making them easy to identify. If light acted only as a wave, then there should be a continuous rainbow created by the prism. Instead, there are discrete lines created by different wavelengths. This is because electrons release specific wavelengths of li ...
Welcome to Physics 112N
... Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
... Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
No Slide Title
... A photon has a frequency of 6.0 x 104 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region? l ...
... A photon has a frequency of 6.0 x 104 Hz. Convert this frequency into wavelength (nm). Does this frequency fall in the visible region? l ...
Modern Physics
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
Line Spectra and the Bohr Model
... Line Spectra and the Bohr Model Limitations of the Bohr Model • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can ha ...
... Line Spectra and the Bohr Model Limitations of the Bohr Model • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can ha ...
Chapter 30: Quantum Physics Chapter 31: Atomic Physics Chapter
... Answers to Even-Numbered Conceptual Questions ...
... Answers to Even-Numbered Conceptual Questions ...
lecture25
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
... If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless ...
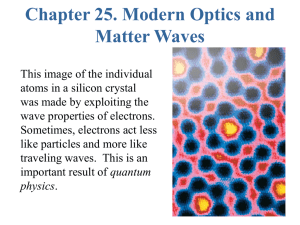
Chapt25_VGO
... Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
... Photo-electric effect (photons) Diffraction of electrons Classical physics was thought to describe the interaction of light with charges until there were discovered too many things that it could not explain. ...
Electronic structure and spectroscopy
... Explanation was given again by Einstein using the quantization introduced by Planck: the light consist of tiny particles which can have energy of hν only. (Note that Planck opposed the use of his „uncompleted” theory!!) ...
... Explanation was given again by Einstein using the quantization introduced by Planck: the light consist of tiny particles which can have energy of hν only. (Note that Planck opposed the use of his „uncompleted” theory!!) ...