Ch4 notes - Midway ISD
... Atomic Orbitals and Quantum numbers • Quantum numbers – specify properties of atomic orbitals and electrons in orbitals • solutions to Schrödinger wave equation ...
... Atomic Orbitals and Quantum numbers • Quantum numbers – specify properties of atomic orbitals and electrons in orbitals • solutions to Schrödinger wave equation ...
Phys202_Exam3_2006.doc
... 26. Why was it so revolutionary? a. it predicted the quantized state of the photon b. it explained the photoelectric effect c. ~ it made matter into a wave whereas it had always been thought of as a particle d. it showed that there was no negative mass state 27. What explicitly did Heisenberg propos ...
... 26. Why was it so revolutionary? a. it predicted the quantized state of the photon b. it explained the photoelectric effect c. ~ it made matter into a wave whereas it had always been thought of as a particle d. it showed that there was no negative mass state 27. What explicitly did Heisenberg propos ...
Slide 1
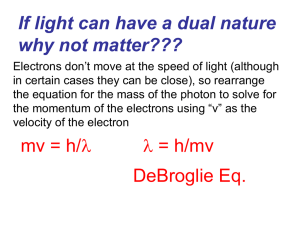
... What would the wavelength of an electron be (9.11 x 10-28 grams) whizzing about it’s orbit in the Bohr model traveling at a speed of 2.0 million meters per second? 3.6 x 10-10 m ...
... What would the wavelength of an electron be (9.11 x 10-28 grams) whizzing about it’s orbit in the Bohr model traveling at a speed of 2.0 million meters per second? 3.6 x 10-10 m ...
Quantum Mechanics
... All matter has wave properties but for large objects, wavelength is too small to be observed Wave nature of electron explains why only some orbits are stable: standing wave must fit in orbit Whole number of wavelengths must equal circumference of orbit ...
... All matter has wave properties but for large objects, wavelength is too small to be observed Wave nature of electron explains why only some orbits are stable: standing wave must fit in orbit Whole number of wavelengths must equal circumference of orbit ...
Section 5-1
... specific amounts called quanta. • Max Planck (1900) Observed - emission of light from hot objects •Concluded - energy is emitted in small, specific amounts (quanta) • A quantum is the minimum amount of energy that can be gained or lost by an atom. • Planck’s constant has a value of 6.626 10–34 J ● ...
... specific amounts called quanta. • Max Planck (1900) Observed - emission of light from hot objects •Concluded - energy is emitted in small, specific amounts (quanta) • A quantum is the minimum amount of energy that can be gained or lost by an atom. • Planck’s constant has a value of 6.626 10–34 J ● ...
5 ELECTRONS IN ATOMS Vocabulary Review Name ___________________________
... 3. the SI unit of frequency ...
... 3. the SI unit of frequency ...
Modern Physics - Tarleton State University
... Man, were they in for a surprise. Several of them actually. Modern physics is the story of these surprises (quantum mechanics and special and general relativity), surprises—revolutions, actually—that have changed the world beyond all recognition. The purpose of this course is to introduce you to all ...
... Man, were they in for a surprise. Several of them actually. Modern physics is the story of these surprises (quantum mechanics and special and general relativity), surprises—revolutions, actually—that have changed the world beyond all recognition. The purpose of this course is to introduce you to all ...
Modern Atomic Theory Notes Sheet
... Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an electron from one orbital to another, ...
... Said Hydrogen’s single electron could only be in certain allowed orbits around the nucleus higher orbit = theorized that electrons existed in distinct orbitals or energy levels around the nucleus, and it took an exact amount of energy or quanta to move an electron from one orbital to another, ...
Quantum Mechanical Model of the Atom
... quantized form • E = hf, where E is energy, h is Planck's constant and f is the frequency of the radiation. ...
... quantized form • E = hf, where E is energy, h is Planck's constant and f is the frequency of the radiation. ...
Chapter 7 NotesAA
... time. In layman’s terms: The more precisely we know a particles position the less we know about its momentum and vice versa. ...
... time. In layman’s terms: The more precisely we know a particles position the less we know about its momentum and vice versa. ...
Electrons in Atoms
... Frequency – units = Hz, s-1 (waves/s); symbol = nu = ; Number of complete waves that pass a point in 1 s time. ...
... Frequency – units = Hz, s-1 (waves/s); symbol = nu = ; Number of complete waves that pass a point in 1 s time. ...
B E , 2012
... a) Discuss the phenomenon of interference of light due to thin film and find the condition of maxima and minima. b) Describe and explain the formation of Newton’s rings in reflected monochromatic light. Prove that in reflected light, diameters of bright rings are proportional to the square-roots of ...
... a) Discuss the phenomenon of interference of light due to thin film and find the condition of maxima and minima. b) Describe and explain the formation of Newton’s rings in reflected monochromatic light. Prove that in reflected light, diameters of bright rings are proportional to the square-roots of ...
Arrangement of Electrons In Atoms
... frequency of electromagnetic radiation 2. Discuss the wave-particle duality of light 3. How the photoelectric effect and line-emission spectrum of hydrogen helped develop the atomic model 4. Describe the Bohr model of the atom ...
... frequency of electromagnetic radiation 2. Discuss the wave-particle duality of light 3. How the photoelectric effect and line-emission spectrum of hydrogen helped develop the atomic model 4. Describe the Bohr model of the atom ...
Problem Set 1 - MIT OpenCourseWare
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
qp2
... product of Planck’s constant h and the frequency of the light wave f, that is, E = hf. This crucial equation relates the wave property of light (its wave frequency) to the particle property of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related ...
... product of Planck’s constant h and the frequency of the light wave f, that is, E = hf. This crucial equation relates the wave property of light (its wave frequency) to the particle property of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related ...
Ch.5 VocabReview
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
... 1. The lowest-energy arrangement of electrons in a subshell is obtained by putting electrons into separate orbitals of the subshell before pairing electrons. ...
D NAME: 1. Which of the following phenomena could not be expla
... Which of the following phenomena could not be explained by classical physics and required a quantum hypothesis in order to make theory agree with experiment? ...
... Which of the following phenomena could not be explained by classical physics and required a quantum hypothesis in order to make theory agree with experiment? ...
Arrangement of Electrons in Atoms I. The Development of a New
... Before the 1900’s, scientists thought light behaved only as waves. This belief changed when it was discovered that light also has particle-like characteristics. This is known as the wave-particle duality of light. ...
... Before the 1900’s, scientists thought light behaved only as waves. This belief changed when it was discovered that light also has particle-like characteristics. This is known as the wave-particle duality of light. ...