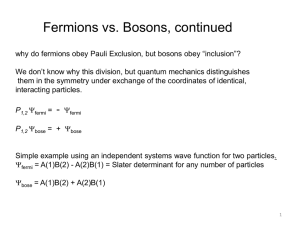
Part 1 Electron Arrangement
... • Heisenberg Uncertainty Principle (1927) – it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. • Schrodinger Wave Equation (1926) - developed an equation that treated electrons in atoms as waves. • Heisenberg and Schrodinger laid the fo ...
... • Heisenberg Uncertainty Principle (1927) – it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. • Schrodinger Wave Equation (1926) - developed an equation that treated electrons in atoms as waves. • Heisenberg and Schrodinger laid the fo ...
Chapter2. Elements of quantum mechanics
... ves. He suggested that since waves exhibit particle-like behavior, then particles should be expected to show wave-like properties. de Broglie suggested that the wavelength of a particle is expressed as = h /p, where p is the momentum of a particle ...
... ves. He suggested that since waves exhibit particle-like behavior, then particles should be expected to show wave-like properties. de Broglie suggested that the wavelength of a particle is expressed as = h /p, where p is the momentum of a particle ...
Quantization of Energy
... • Although the Rayleigh-Jeans law works for low frequencies, it diverges at high ν • This divergence for high frequencies is called the ultraviolet catastrophe. ...
... • Although the Rayleigh-Jeans law works for low frequencies, it diverges at high ν • This divergence for high frequencies is called the ultraviolet catastrophe. ...
Schrödinger`s Wave Mechanical Model
... o Delocalized-position in space cannot be specified. No intermingling between the two This all changed with the start of the 20th century and the work of Max Planck. Planck – Observed radiations emitted from solid bodies heated to incandescence (glowing). This could not be explained by classical p ...
... o Delocalized-position in space cannot be specified. No intermingling between the two This all changed with the start of the 20th century and the work of Max Planck. Planck – Observed radiations emitted from solid bodies heated to incandescence (glowing). This could not be explained by classical p ...
Document
... the experiment fit. He developed a quantization theory to predict the value h. – “lucky artifact of more fundamental reality yet to be discovered” ...
... the experiment fit. He developed a quantization theory to predict the value h. – “lucky artifact of more fundamental reality yet to be discovered” ...
Elec Structure of Atom
... high energy state to a low energy state; light can be absorbed to excite the electron from a low energy state to a high energy state. The frequency of light emitted or absorbed must be such that hv=the difference in energy between two allowed states of the atom. ...
... high energy state to a low energy state; light can be absorbed to excite the electron from a low energy state to a high energy state. The frequency of light emitted or absorbed must be such that hv=the difference in energy between two allowed states of the atom. ...
Lecture 24 (7.1-7.2)
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
Quantum Physics - Particle Physics and Particle Astrophysics
... – energy of electrons depends on frequency of light, KE = hf – w – rate of emission (current) depends on intensity of light – this is inexplicable if light is a continuous wave, but simple to understand if it is composed of particles (photons) of energy hf ...
... – energy of electrons depends on frequency of light, KE = hf – w – rate of emission (current) depends on intensity of light – this is inexplicable if light is a continuous wave, but simple to understand if it is composed of particles (photons) of energy hf ...
Chp.23 Outline - Redlands High School
... better in the particle or wave nature of light? What does an electron volt measure? How many Joules are in an electron volt? 2) What is a blackbody? As the temperature of a blackbody increases what does classical mechanics predict for the intensity of different wavelengths of EM radiation that are e ...
... better in the particle or wave nature of light? What does an electron volt measure? How many Joules are in an electron volt? 2) What is a blackbody? As the temperature of a blackbody increases what does classical mechanics predict for the intensity of different wavelengths of EM radiation that are e ...
Development of the Model of the Atom
... by proposing that radiation is absorbed by matter in whole numbers of photons. In order for an electron to be ejected, the electron must be struck by a single photon possessing a minimum amount of energy (which corresponds to the frequency). ...
... by proposing that radiation is absorbed by matter in whole numbers of photons. In order for an electron to be ejected, the electron must be struck by a single photon possessing a minimum amount of energy (which corresponds to the frequency). ...
Thursday, 1/29/09 - Liberty Union High School District
... frequency was required to eject electron from metal •Problem-wave theory of light predicted light of any frequency could eject electron ...
... frequency was required to eject electron from metal •Problem-wave theory of light predicted light of any frequency could eject electron ...
Electron configuration Jeopardy
... 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spectrum is like the rainbow. You could look out the window to see that. Line spectrum is certain colors show up in lines. You could pass electricity through an ...
... 500 – What is the difference between a continuous spectrum and a line spectrum and give an example of where you could find each. Continuous spectrum is like the rainbow. You could look out the window to see that. Line spectrum is certain colors show up in lines. You could pass electricity through an ...
1. Crystal Properties and Growth of Semiconductors
... Shortest Course in Quantum Mechanics, Cont. Heisenberg Uncertainty Principle (Nobel prize 1932 for the creation of quantum mechanics) The more precise you know the position of a particle , the less precise you know the momentum of the ...
... Shortest Course in Quantum Mechanics, Cont. Heisenberg Uncertainty Principle (Nobel prize 1932 for the creation of quantum mechanics) The more precise you know the position of a particle , the less precise you know the momentum of the ...
You may recall the formula: V = W/q Potential difference between
... 1) Yellow light with a frequency of 5.43 x 1014 Hz strikes a cesium surface. If the photoelectric work function of cesium is 3.42 x 1019 J, what is the maximum velocity a photoelectron emitted from the surface can have? (mass of electron = 9.11 x 1031 kg) hf − φ = ½ mv2 (6.626x10-34 Js)(5.4 ...
... 1) Yellow light with a frequency of 5.43 x 1014 Hz strikes a cesium surface. If the photoelectric work function of cesium is 3.42 x 1019 J, what is the maximum velocity a photoelectron emitted from the surface can have? (mass of electron = 9.11 x 1031 kg) hf − φ = ½ mv2 (6.626x10-34 Js)(5.4 ...
Lecture #3
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
3.4oquantum.4u
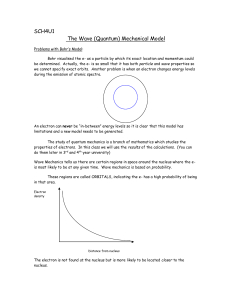
... The study of quantum mechanics is a branch of mathematics which studies the properties of electrons. In this class we will use the results of the calculations. (You can do them later in 3rd and 4th year university) Wave Mechanics tells us there are certain regions in space around the nucleus where t ...
... The study of quantum mechanics is a branch of mathematics which studies the properties of electrons. In this class we will use the results of the calculations. (You can do them later in 3rd and 4th year university) Wave Mechanics tells us there are certain regions in space around the nucleus where t ...