Double-Slit Experiment
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
... Energy is quantized. It can occur only in discrete units called ______________. de Broglie: Corrected equation to account for relationship between mass and wavelength: m= de Broglie equation: ...
Read Notes #1 - Faculty Website Listing
... electron) can only be explained by the particle representation while others can only be explained with the wave picture. ...
... electron) can only be explained by the particle representation while others can only be explained with the wave picture. ...
Ch. 5 Outline
... KC 18 How is the change in electron energy related to the frequency of light emitted in atomic transitions? ...
... KC 18 How is the change in electron energy related to the frequency of light emitted in atomic transitions? ...
Atomic Physics 4
... circular or elliptical paths would feel a centripetal force and would therefore have centripetal acceleration. – But accelerating charges produce electric magnetic radiation, therefore these electrons should continuously emit energy, slow down, and eventually collapse into the nucleus. – Obviously, ...
... circular or elliptical paths would feel a centripetal force and would therefore have centripetal acceleration. – But accelerating charges produce electric magnetic radiation, therefore these electrons should continuously emit energy, slow down, and eventually collapse into the nucleus. – Obviously, ...
ppt - HEP Educational Outreach
... Early theoretical models to explain Blackbody spectrum had divergence at low wavelength: “ultraviolet catastrophe”. ...
... Early theoretical models to explain Blackbody spectrum had divergence at low wavelength: “ultraviolet catastrophe”. ...
honors-chapter6-reading
... 2. Explain the relationship between quantum of energy and Planck’s constant. Be sure to include the equation for energy in your discussion. 3. Explain the photoelectric effect and role of photons. 4. Why is light (electromagnetic radiation) described as having both wavelike and particle-like charact ...
... 2. Explain the relationship between quantum of energy and Planck’s constant. Be sure to include the equation for energy in your discussion. 3. Explain the photoelectric effect and role of photons. 4. Why is light (electromagnetic radiation) described as having both wavelike and particle-like charact ...
Ideas of Modern Physics
... 1. A scientist is trying to eject electrons from a metal by shining a light on it, but none are coming out. To eject electrons, she should change the light by… a. decreasing the frequency b. increasing the frequency c. increasing the intensity d. increasing the wavelength e. asking Einstein 2. A bet ...
... 1. A scientist is trying to eject electrons from a metal by shining a light on it, but none are coming out. To eject electrons, she should change the light by… a. decreasing the frequency b. increasing the frequency c. increasing the intensity d. increasing the wavelength e. asking Einstein 2. A bet ...
DOC - 嘉義大學
... (c) What speed (in units of c) is the neutron moving in this case? (d) What is the neutron’s momentum in unit of MeV/c? 2. Suppose that light of total intensity 1.0 W/cm2 falls on a clean zinc (Zn) sample which the area is 1.01.0 cm2. Assume that the Zn sample reflects 95% of the light (absorbs 5% ...
... (c) What speed (in units of c) is the neutron moving in this case? (d) What is the neutron’s momentum in unit of MeV/c? 2. Suppose that light of total intensity 1.0 W/cm2 falls on a clean zinc (Zn) sample which the area is 1.01.0 cm2. Assume that the Zn sample reflects 95% of the light (absorbs 5% ...
S
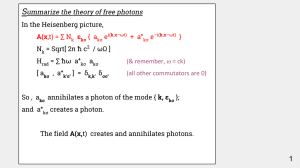
... A(x,t) = ∑ Nk εkσ { akσ ei(k.x–ωt) + a+kσ e–i(k.x–ωt) } Nk = Sqrt[ 2π ħ c2 / ωΩ ] Hrad = ∑ ħω a+kσ akσ ...
... A(x,t) = ∑ Nk εkσ { akσ ei(k.x–ωt) + a+kσ e–i(k.x–ωt) } Nk = Sqrt[ 2π ħ c2 / ωΩ ] Hrad = ∑ ħω a+kσ akσ ...
Superconcepts
... ix. De Broglie found that electrons behaved as waves in diffraction experiments in 1924. x. Schrödinger showed that atomic wave spectra (electrons) could be predicted by wave functions in 1926. xi. Heisinger found that atomic properties were ‘indeterminant’ or uncertain in 1927. xii. When the double ...
... ix. De Broglie found that electrons behaved as waves in diffraction experiments in 1924. x. Schrödinger showed that atomic wave spectra (electrons) could be predicted by wave functions in 1926. xi. Heisinger found that atomic properties were ‘indeterminant’ or uncertain in 1927. xii. When the double ...
Section 5-2
... • All moving particles behave like waves • Wave characteristics decrease as mass increases ...
... • All moving particles behave like waves • Wave characteristics decrease as mass increases ...
Document
... a. a type of matter c. a type of energy b. a type of sound wave d. a type of water wave 3. How is light different from other kinds of waves? Light does not require matter through which to travel. Other kinds of waves must travel through matter. 4. A wave that consists of changing electric and magnet ...
... a. a type of matter c. a type of energy b. a type of sound wave d. a type of water wave 3. How is light different from other kinds of waves? Light does not require matter through which to travel. Other kinds of waves must travel through matter. 4. A wave that consists of changing electric and magnet ...
Physics116_L31
... Photon collisions are same as for particle with momentum p and energy E Energy lost knocking an electron out of atom is given by same equation as for particles with mass – observe a longer wavelength afterward (lower E) ...
... Photon collisions are same as for particle with momentum p and energy E Energy lost knocking an electron out of atom is given by same equation as for particles with mass – observe a longer wavelength afterward (lower E) ...
Chapter 7: Electrons in Atoms Electromagnetic Radiation
... • ne- a I # of e- depends on intensity (Absence of Lag time. Current flow is immediate. The metal does not “accumulate” energy to eject the electron as predicted by wave theory) • Ek α ν kinetic energy depends on frequency (Wave theory says that amplitude, not frequency is responsible… so any colo ...
... • ne- a I # of e- depends on intensity (Absence of Lag time. Current flow is immediate. The metal does not “accumulate” energy to eject the electron as predicted by wave theory) • Ek α ν kinetic energy depends on frequency (Wave theory says that amplitude, not frequency is responsible… so any colo ...
quantum1
... Assuming this particle exists, at any given time it must be somewhere. This requirement can be expressed mathematically as: If you search from hither to yon ...
... Assuming this particle exists, at any given time it must be somewhere. This requirement can be expressed mathematically as: If you search from hither to yon ...
Chapter 7
... • Conclusion: electrons have wave-like properties. • The Davisson-Germer experiment has been repeated with other particles, including a particles and molecular H. ...
... • Conclusion: electrons have wave-like properties. • The Davisson-Germer experiment has been repeated with other particles, including a particles and molecular H. ...
Review for Chapter 7
... This spectrum includes gamma rays, X-rays, ultraviolet rays, visible light waves, infrared waves, microwaves, and radio and TV waves (see Figure 7.4). 9. Visible light ranges from a wavelength of approximately 400 nm (violet) to about 700 nm (red). 10. Einstein figured out how to explain the photoel ...
... This spectrum includes gamma rays, X-rays, ultraviolet rays, visible light waves, infrared waves, microwaves, and radio and TV waves (see Figure 7.4). 9. Visible light ranges from a wavelength of approximately 400 nm (violet) to about 700 nm (red). 10. Einstein figured out how to explain the photoel ...
Electric Potential
... 1801: Through use of the Double-Slit Experiment, the wave properties of light were first experimentally shown to exist. Experiment demonstrated that light undergoes interference and diffraction in much the same way that water and sound waves do. Used source of monochromatic light to eliminate the pr ...
... 1801: Through use of the Double-Slit Experiment, the wave properties of light were first experimentally shown to exist. Experiment demonstrated that light undergoes interference and diffraction in much the same way that water and sound waves do. Used source of monochromatic light to eliminate the pr ...
Chapter 4
... – connect wave and particle nature of matter using a relationship that applies to photons: = h/p where p is the momentum of the particle (p = mass times velocity). ...
... – connect wave and particle nature of matter using a relationship that applies to photons: = h/p where p is the momentum of the particle (p = mass times velocity). ...