LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of separation of variables to break up Schrodinger equation for a rigid rotor into ...
... Derive time independent Schrodinger wave equation from time dependent equation. The force constant for H79Br is 392 Nm-1. Calculate the fundamental vibrational frequency and zero point energy of H79Br. Use the method of separation of variables to break up Schrodinger equation for a rigid rotor into ...
3.3 Review Name________________________________ Period_______Date_____________________
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
Document
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
Introduction to Electromagnetism
... •This does not mean that the system was at X before the measurement - it is not meaningful to say it was localized at all before the measurement. ...
... •This does not mean that the system was at X before the measurement - it is not meaningful to say it was localized at all before the measurement. ...
Document
... What is the de Broglie wavelength of a 50 kg person traveling at 15 m/s? (h = 6.6 x 10-34 J s) comparable to spacing ...
... What is the de Broglie wavelength of a 50 kg person traveling at 15 m/s? (h = 6.6 x 10-34 J s) comparable to spacing ...
Quiz 1 Key
... Describe what the emission spectra of a hydrogen atom looks like compared to that of white light and report what this indicated about the energy of the electrons around the hydrogen atom. White light has all of the colors and therefore wavelengths present. The emission spectrum of hydrogen had only ...
... Describe what the emission spectra of a hydrogen atom looks like compared to that of white light and report what this indicated about the energy of the electrons around the hydrogen atom. White light has all of the colors and therefore wavelengths present. The emission spectrum of hydrogen had only ...
Energy
... light. • Photoelectrons are not released below a certain frequency, regardless of intensity of light. • The release of photoelectrons is instantaneous, even in very feeble light, provided the frequency is above the ...
... light. • Photoelectrons are not released below a certain frequency, regardless of intensity of light. • The release of photoelectrons is instantaneous, even in very feeble light, provided the frequency is above the ...
CLASSICAL-QUANTUM CORRESPONDENCE AND WAVE PACKET SOLUTIONS OF THE DIRAC
... well-known relation E = ~ω in the specific form H = ~W , where H is the classical Hamiltonian of a particle and W is the dispersion relation of the sought-for wave equation. We derive the expression of H in a curved space-time with an electromagnetic field. Then we derive the Dirac equation from fac ...
... well-known relation E = ~ω in the specific form H = ~W , where H is the classical Hamiltonian of a particle and W is the dispersion relation of the sought-for wave equation. We derive the expression of H in a curved space-time with an electromagnetic field. Then we derive the Dirac equation from fac ...
Quantum Physics in a Nutshell
... Remember back to the Special Theory of Relativity… • Remember how the Michelson-Morely experiment was trying to prove that “ether” existed? This was because they thought light behaved as a mechanical wave (needed a medium for the energy to travel through). • Then, once this was disproven, it was th ...
... Remember back to the Special Theory of Relativity… • Remember how the Michelson-Morely experiment was trying to prove that “ether” existed? This was because they thought light behaved as a mechanical wave (needed a medium for the energy to travel through). • Then, once this was disproven, it was th ...
Chapter 4
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
... Rutherford model…It did not answer: Where the e- were located in the space outside the nucleus Why the e- did not crash into the nucleus Why atoms produce spectra at specific wavelengths ...
Waves, incl. Electromagnetic Waves, Light
... disappears if either source A or source B is turned off. This implies a seemingly paradoxical situation: adding a 2nd source reduces the wave effect at certain locations (marked 0)! Precisely the nature of interference, though. Also very important: the two sources A & B must be synchronized, i.e. if ...
... disappears if either source A or source B is turned off. This implies a seemingly paradoxical situation: adding a 2nd source reduces the wave effect at certain locations (marked 0)! Precisely the nature of interference, though. Also very important: the two sources A & B must be synchronized, i.e. if ...
powerpoint
... A surface of metal illuminated by light ejects electrons. The kinetic energy of the electrons is proportional to the frequency of the impinging light. ...
... A surface of metal illuminated by light ejects electrons. The kinetic energy of the electrons is proportional to the frequency of the impinging light. ...
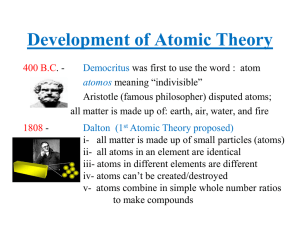
Development of Atomic Theory
... - experimental evidence from line spectra of elements supported this. Energy is released (emission) or absorbed (absorption) by electrons at certain ...
... - experimental evidence from line spectra of elements supported this. Energy is released (emission) or absorbed (absorption) by electrons at certain ...
Momentum Do photons carry momentum ? DeBroglie`s Relation
... = 276 nm (in the UV range) Any frequency lower than the cut-off (or any wavelength greater than the cut-off value) will NOT eject electrons from the metal. From Einstein’s equation: hf = W + e*Vstop , we can see that the straight line of the Vstop vs f graph should have a slope of (h/e) . ...
... = 276 nm (in the UV range) Any frequency lower than the cut-off (or any wavelength greater than the cut-off value) will NOT eject electrons from the metal. From Einstein’s equation: hf = W + e*Vstop , we can see that the straight line of the Vstop vs f graph should have a slope of (h/e) . ...
Quantum Physics 2 - More About
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
... prediction, first made in the late 19th century, that an IDEAL BLACK BODY at thermal equilibrium will emit radiation with INFINITE POWER. Max Planck resolved this issue by postulating that electromagnetic energy did not follow the classical description, but could only oscillate or be emitted in DISC ...
Slide 1
... Therefore, as l decreases, n increases C = speed of light, (186,000 miles/s, or 299,792,458 m/ s) ...
... Therefore, as l decreases, n increases C = speed of light, (186,000 miles/s, or 299,792,458 m/ s) ...
WP1
... pattern? Yes. Somehow the single electrons are interfering with themselves! What! How? Does a single electron go through both slits (to cause the interference)? How can an electron that causes a localized flash on the absorbing screen go through both slits? Can an electron be in two places at once? ...
... pattern? Yes. Somehow the single electrons are interfering with themselves! What! How? Does a single electron go through both slits (to cause the interference)? How can an electron that causes a localized flash on the absorbing screen go through both slits? Can an electron be in two places at once? ...