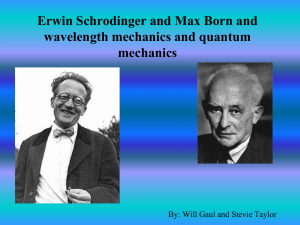double-slit worksheet
... a) How fast were these electrons moving? b) How fast is that compared to the speed of light? 42 %. c) How long would they take to cross the 1 m apparatus? d) What was the wavelength of these electrons? e) Should relativistic equations be used? f) How was the slit set-up in this experiment different ...
... a) How fast were these electrons moving? b) How fast is that compared to the speed of light? 42 %. c) How long would they take to cross the 1 m apparatus? d) What was the wavelength of these electrons? e) Should relativistic equations be used? f) How was the slit set-up in this experiment different ...
Quantum Notes
... light’s characteristics; Albert Einstein proposed in 1905 that light has a dual nature (wave-like and particle-like) • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be gained or lost by an atom ...
... light’s characteristics; Albert Einstein proposed in 1905 that light has a dual nature (wave-like and particle-like) • Matter can gain or lose energy only in small, specific amounts called quanta •A quantum is the minimum amount of energy that can be gained or lost by an atom ...
Adobe Acrobat file ()
... energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different energies, Equation 27.15 tells us that the particles do not have the same frequency. ...
... energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different energies, Equation 27.15 tells us that the particles do not have the same frequency. ...
Quantum Mechanics
... Wavefunction ψ (Psi) describes a quantum mechanical system. The nature of a system can be described by probabilistic values; probability of an event is equal to the square of the amplitude of the wavefunction (|ψ|²). Impossible to know all properties of a system at the same time, each must be given ...
... Wavefunction ψ (Psi) describes a quantum mechanical system. The nature of a system can be described by probabilistic values; probability of an event is equal to the square of the amplitude of the wavefunction (|ψ|²). Impossible to know all properties of a system at the same time, each must be given ...
Particle wavelength, Rutherford scattering
... shown here on a bacterium). This picture is the output of an “electron microscope” ...
... shown here on a bacterium). This picture is the output of an “electron microscope” ...
Evolution of the Atomic Theory
... • 1. a large majority of alpha particles passed directly through the foil. • 2. few particles were deflected when shot at the foil. • 3. rarely, one particle would come back almost directly at the alpha source ...
... • 1. a large majority of alpha particles passed directly through the foil. • 2. few particles were deflected when shot at the foil. • 3. rarely, one particle would come back almost directly at the alpha source ...
introduction to atomic structure
... Bohr’s Postulates: •Electron moves in circular orbits around the nucleus. •Electron can possess only certain energy values corresponding to the orbit. •Electron can “jump” from one orbit to another, the energy difference will be emitted or absorbed in the form of light quanta. ...
... Bohr’s Postulates: •Electron moves in circular orbits around the nucleus. •Electron can possess only certain energy values corresponding to the orbit. •Electron can “jump” from one orbit to another, the energy difference will be emitted or absorbed in the form of light quanta. ...
$doc.title
... ⇒ pattern results from addition of many particles! - Pattern gives probability of any single particle location ...
... ⇒ pattern results from addition of many particles! - Pattern gives probability of any single particle location ...
Physics116_L35
... Review: what you’ve learned since exam 2 • Superposition and Interference: overlapping waves’ amplitudes add • Young's Two-Slit Experiment: proof that light = waves; regularly spaced bright and dark fringes • Interference in Reflected Waves: phase shift in reflected wave when going from lower to hi ...
... Review: what you’ve learned since exam 2 • Superposition and Interference: overlapping waves’ amplitudes add • Young's Two-Slit Experiment: proof that light = waves; regularly spaced bright and dark fringes • Interference in Reflected Waves: phase shift in reflected wave when going from lower to hi ...
Quantum Mechanics and the Bohr Model - slater science
... • Using the line as the midpoint draw two waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
... • Using the line as the midpoint draw two waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
Presentation
... interference pattern is still produced one “particle” at a time. =h/p “It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency.” ...
... interference pattern is still produced one “particle” at a time. =h/p “It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency.” ...
wave function - Purdue Physics
... constructive and destructive interference at certain locations on the screen • The experiment also shows aspects of particle-like behavior since the electrons arrive one at a time at the screen, and also don’t just go in straight lines. • In principle, you can slowly build up an interference pattern ...
... constructive and destructive interference at certain locations on the screen • The experiment also shows aspects of particle-like behavior since the electrons arrive one at a time at the screen, and also don’t just go in straight lines. • In principle, you can slowly build up an interference pattern ...
Lecture 24: Quantum mechanics
... Non-classical Explanation: Planck hypothesis. Planck assumed that the radiating substance was composed of electric dipoles that acted as simple harmonic oscillators. His suggestion was as follows: The energy of an oscillator must be discrete. E = n h where n is an integer, h is a constant of propo ...
... Non-classical Explanation: Planck hypothesis. Planck assumed that the radiating substance was composed of electric dipoles that acted as simple harmonic oscillators. His suggestion was as follows: The energy of an oscillator must be discrete. E = n h where n is an integer, h is a constant of propo ...
Mathematical Methods of Physics – Fall 2010 – Dr
... photons have energy E = h = hc/ and momentum p = h/ can be created or destroyed when radiation (e.g. particles) are emitted or absorbed can have particle-like collisions with other particles such as electrons Light also exhibits wave-like properties such as interference and diffraction. QM: ...
... photons have energy E = h = hc/ and momentum p = h/ can be created or destroyed when radiation (e.g. particles) are emitted or absorbed can have particle-like collisions with other particles such as electrons Light also exhibits wave-like properties such as interference and diffraction. QM: ...
Quantum eraser
... pattern? Let us examine a simple information erasing scheme by modeling our system with qubits. Say we have a 2 slit apparatus in which we can mark the path the particle takes using qubits A and B each marking whether the particle passed through the appropriate slit or not. Another qubit D is the de ...
... pattern? Let us examine a simple information erasing scheme by modeling our system with qubits. Say we have a 2 slit apparatus in which we can mark the path the particle takes using qubits A and B each marking whether the particle passed through the appropriate slit or not. Another qubit D is the de ...
Lecture 24 (7.1-7.2)
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
... The particle nature of light • Blackbody radiation – light emitted from solid objects heated to incandescence – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained th ...
Quantum Potpourri
... and the Superposition Principle Electrons in atoms or molecules are characterized by their entire distributions, called wave functions or orbitals, rather than by instantaneous positions and velocities: an electron may be considered always to be, with appropriate probability, at all points of its di ...
... and the Superposition Principle Electrons in atoms or molecules are characterized by their entire distributions, called wave functions or orbitals, rather than by instantaneous positions and velocities: an electron may be considered always to be, with appropriate probability, at all points of its di ...
CHEM-UA 127: Advanced General Chemistry I
... of the particle, itself, rather than a limit on our ability to perform an accurate enough measurement. This idea of a fundamental limit to what is knowable about a quantum particle (or collection of quantum particles) was put forth by the physicist Werner Heisenberg in 1927. His principle is now one ...
... of the particle, itself, rather than a limit on our ability to perform an accurate enough measurement. This idea of a fundamental limit to what is knowable about a quantum particle (or collection of quantum particles) was put forth by the physicist Werner Heisenberg in 1927. His principle is now one ...