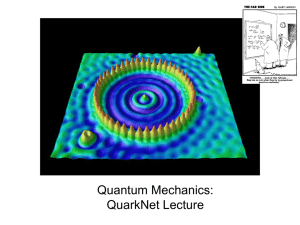
Slide 1 - s3.amazonaws.com
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
5.0. Wave Mechanics
... 5.0. Wave Mechanics One cornerstone of quantum theory is the particle-wave duality [the other is the principle of uncertainty]. For example, in optical phenomena such as diffraction and interference, light behaves like waves. In collision processes such as photo-electric and Compton effects, light b ...
... 5.0. Wave Mechanics One cornerstone of quantum theory is the particle-wave duality [the other is the principle of uncertainty]. For example, in optical phenomena such as diffraction and interference, light behaves like waves. In collision processes such as photo-electric and Compton effects, light b ...
Two-electron Interference
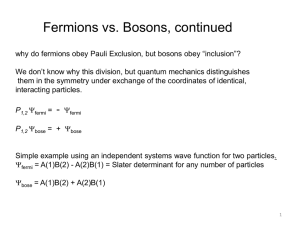
... quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interference between two particles is a direct result of quantum exchange statistics. Such int ...
... quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interference between two particles is a direct result of quantum exchange statistics. Such int ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... The theory was found to be extremely successful in describing nature (see rest of the course), but as two of its fathers put it: “To try and stop all attempts to pass beyond the present viewpoint of quantum physics could be very dangerous for the progress of science and would furthermore be contrary ...
... The theory was found to be extremely successful in describing nature (see rest of the course), but as two of its fathers put it: “To try and stop all attempts to pass beyond the present viewpoint of quantum physics could be very dangerous for the progress of science and would furthermore be contrary ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... • Quantum mechanics was born out of several experimental problems such as the UV catastrophe, and the quantized absorption and emission spectrum of the atoms (light and Frank-Hertz) • First good predictions were achieved when one assumed quantization of the atomic oscillations in the bulk (Plank), a ...
... • Quantum mechanics was born out of several experimental problems such as the UV catastrophe, and the quantized absorption and emission spectrum of the atoms (light and Frank-Hertz) • First good predictions were achieved when one assumed quantization of the atomic oscillations in the bulk (Plank), a ...
Lecture #3
... interference pattern of single electrons. Numbers of electrons are 10 (a), 200 (b), 6000 (c), 40000 (d), 140000 (e). (Provided with kind permission of Dr. AkiraTonomura.) Electron or photon interference is a single particle phenomenon! ...
... interference pattern of single electrons. Numbers of electrons are 10 (a), 200 (b), 6000 (c), 40000 (d), 140000 (e). (Provided with kind permission of Dr. AkiraTonomura.) Electron or photon interference is a single particle phenomenon! ...
Modern physics 2330
... Q1 Indicate (√ ) for true statement and (× ) for false statement. 1- ( ) A black body is defined as an object that absorbs all the electromagnetic radiation falling on it and consequently appears black. 2- ( ) In certain situations light exhibits wave properties like diffraction and Compton Effect. ...
... Q1 Indicate (√ ) for true statement and (× ) for false statement. 1- ( ) A black body is defined as an object that absorbs all the electromagnetic radiation falling on it and consequently appears black. 2- ( ) In certain situations light exhibits wave properties like diffraction and Compton Effect. ...
Linear Circuit Analysis with Reactive Components
... Solving the Schrödinger Equation on a 2D Lattice in Quantum Wave Interference (QWI) PhET Sam Reid Quantum Wave Interference allows the user to visualize the propagation of a wavefunction in the presence of potential barriers and detectors. We implement a 2D Richardson algorithm[1], a local propagati ...
... Solving the Schrödinger Equation on a 2D Lattice in Quantum Wave Interference (QWI) PhET Sam Reid Quantum Wave Interference allows the user to visualize the propagation of a wavefunction in the presence of potential barriers and detectors. We implement a 2D Richardson algorithm[1], a local propagati ...
history
... detect the passage of a particle through either of the Double-slit experiment is one of the basic slits, its wave function collapses and it passes through experiments of quantum mechanics that proves waveonly one of the slits as a classical particle . As particle duality. We would like to demonstrat ...
... detect the passage of a particle through either of the Double-slit experiment is one of the basic slits, its wave function collapses and it passes through experiments of quantum mechanics that proves waveonly one of the slits as a classical particle . As particle duality. We would like to demonstrat ...
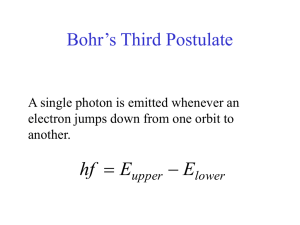
Bohr´s Third Postulate
... 3. If a metal surface is illuminated by light at a single frequency, why don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons dep ...
... 3. If a metal surface is illuminated by light at a single frequency, why don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons dep ...
File
... 3. A water wave passes through two slits. Which pattern best matches the amplitude of the resulting wave? ...
... 3. A water wave passes through two slits. Which pattern best matches the amplitude of the resulting wave? ...
De Broglie and Heisenberg
... The modern double-slit experiment is a demonstration that light and matter can display characteristics of both classically defined waves and particles; moreover, it displays the fundamentally probabilistic nature of quantum mechanical phenomena. A simpler form of the double-slit experiment was perfo ...
... The modern double-slit experiment is a demonstration that light and matter can display characteristics of both classically defined waves and particles; moreover, it displays the fundamentally probabilistic nature of quantum mechanical phenomena. A simpler form of the double-slit experiment was perfo ...
Atomic Diffraction Dr. Janine Shertzer College of the Holy Cross
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...