* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Evolution of the Atomic Theory
Introduction to quantum mechanics wikipedia , lookup
Nuclear structure wikipedia , lookup
Minimal Supersymmetric Standard Model wikipedia , lookup
Future Circular Collider wikipedia , lookup
Renormalization wikipedia , lookup
Electric charge wikipedia , lookup
Mathematical formulation of the Standard Model wikipedia , lookup
ALICE experiment wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Weakly-interacting massive particles wikipedia , lookup
Grand Unified Theory wikipedia , lookup
Identical particles wikipedia , lookup
Compact Muon Solenoid wikipedia , lookup
Standard Model wikipedia , lookup
Double-slit experiment wikipedia , lookup
ATLAS experiment wikipedia , lookup
Electron scattering wikipedia , lookup
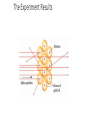
Evolution of the Atomic Theory Visualizing the ATOM Another Addition • 1911 – Ernest Rutherford • Gold Foil Experiment • Bombarded a piece of gold foil with alpha particles (positively charged particles). The Experiment Setup The Experiment Results Rutherford’s Experiment • Gold Foil Experiment • Observations: • 1. a large majority of alpha particles passed directly through the foil. • 2. few particles were deflected when shot at the foil. • 3. rarely, one particle would come back almost directly at the alpha source More progress! The Atomic Model • Conclusions: • 1. Most of an atom consists of empty space. • 2. There lies a condensed positive mass in an element. • Rutherford discovered the positively charged atomic nucleus. Rutherford’s Atomic Model Discovery of the Proton • Although Rutherford was credited with the positive nucleus, Eugene Goldstein was credited with the discovery of protons. • “Anode beam” – similar to Thomson Up to this point in time… • Mass ratio tests were experimented on these subatomic particles to tell us: Subatomic Particle Electric Charge Actual Mass Relative Mass (amu) Electron -1 9.11 x 10-31 kg 0 Proton +1 1.67 x 10-27 kg 1 The Last Addition • 1932 – James Chadwick • There had to be something else!! • 1. This particle had to be electrically neutral. • 2. This particle had to contribute to the atoms mass. • Chadwick would later name this mystery particle the neutron; which is located in the nucleus of an atom. Chadwick’s Contribution Subatomic Particles: Subatomic Particle Electric Charge Actual Mass Relative Mass (amu) Electron -1 9.11 x 10-31 kg 0 Proton +1 1.67 x 10-27 kg 1 Neutron 0 1.67 x 10-27 kg 1