Novel Substrates for Fluorescence-based Protein Tyrosine Kinase
... the microplates were sealed with TopSeal-A™ (PKI) during that time. The fluorescence signals were read using an excitation filter of 320 nm and an emission filter of 665 nm on an EnVision® Multilabel Reader (PKI). The final assay volume was 20 µL. Determination of kinase concentration – 0 to 30 nM o ...
... the microplates were sealed with TopSeal-A™ (PKI) during that time. The fluorescence signals were read using an excitation filter of 320 nm and an emission filter of 665 nm on an EnVision® Multilabel Reader (PKI). The final assay volume was 20 µL. Determination of kinase concentration – 0 to 30 nM o ...
File
... higher concentrations than other amino acids in most tissues. • In skeletal muscle, excess amino groups are generally transferred to pyruvate to form alanine, another important molecule in the transport of amino groups to the liver. ...
... higher concentrations than other amino acids in most tissues. • In skeletal muscle, excess amino groups are generally transferred to pyruvate to form alanine, another important molecule in the transport of amino groups to the liver. ...
RNA/DNA catalysts
... introns, RNase P, small self-cleaving), what reactions they perform, know basics of their secondary and tertiary structure, requirements for cofactors/metals/proteins/ATP Know details of glmS ribozyme self-cleavage Understand use of ribozymes as therapeutics In vitro selection - understand the proce ...
... introns, RNase P, small self-cleaving), what reactions they perform, know basics of their secondary and tertiary structure, requirements for cofactors/metals/proteins/ATP Know details of glmS ribozyme self-cleavage Understand use of ribozymes as therapeutics In vitro selection - understand the proce ...
Experimental and Computational Evidence of Metal‑O2 Activation
... upon exposure to the H2O2 product of enzyme turnover. Previous works have neglected this reaction and assumed that neither CoII nor CuII undergoes a change in redox state during enzyme catalysis.32 The Ered in eq 2 may involve complex internal redox chemistry in certain copper amine oxidases,33−36 w ...
... upon exposure to the H2O2 product of enzyme turnover. Previous works have neglected this reaction and assumed that neither CoII nor CuII undergoes a change in redox state during enzyme catalysis.32 The Ered in eq 2 may involve complex internal redox chemistry in certain copper amine oxidases,33−36 w ...
Lipid Breakdown - Rose
... Odd-numbered fatty acids Fatty acids with odd numbers of carbons are found in some marine animals, in many herbivores, in microorganisms, and in plants. These fatty acids are subjected to β-oxidation in the same way as fatty acids with even numbers of carbons. However, the final β-oxidation spiral r ...
... Odd-numbered fatty acids Fatty acids with odd numbers of carbons are found in some marine animals, in many herbivores, in microorganisms, and in plants. These fatty acids are subjected to β-oxidation in the same way as fatty acids with even numbers of carbons. However, the final β-oxidation spiral r ...
Ch 9 Cellular respiration
... in inner membrane of mitochondrion has increased surface area (cristae) for lots of ETCs mostly made of proteins multiprotein complexes #IIV prosthetic gps. are attached to these proteins nonprotein needed for catalysis by enzymes during chainelectron carriers alternate between reduc ...
... in inner membrane of mitochondrion has increased surface area (cristae) for lots of ETCs mostly made of proteins multiprotein complexes #IIV prosthetic gps. are attached to these proteins nonprotein needed for catalysis by enzymes during chainelectron carriers alternate between reduc ...
Can sugars be produced from fatty acids? A test case for pathway
... The publisher regrets that Figure 3 was incorrect and the correct Figure 3 is included in the full article below. ...
... The publisher regrets that Figure 3 was incorrect and the correct Figure 3 is included in the full article below. ...
Solving Biochemistry`s Biggest Mystery: How We Produce Energy
... The harder problem, however, has been to elucidate the chemical process of how we convert food into energy. Even today, the eyes of many biochemists glaze over when the topic turns to the movement of electrons along the respiratory chain to produce energy. A good way to impress other biochemists is ...
... The harder problem, however, has been to elucidate the chemical process of how we convert food into energy. Even today, the eyes of many biochemists glaze over when the topic turns to the movement of electrons along the respiratory chain to produce energy. A good way to impress other biochemists is ...
Formation of Benzoic Acid and
... experiments on the formation of p-hydroxybenzoic acid from L-tyrosine in A. nidulans. As the exo genously supplied intermediate, which competes with the complex, is 3H-labelled and the substrate for the complex is 14C-labelled, a low ratio 3H /14C stands for a tightly coupled reaction. The respecti ...
... experiments on the formation of p-hydroxybenzoic acid from L-tyrosine in A. nidulans. As the exo genously supplied intermediate, which competes with the complex, is 3H-labelled and the substrate for the complex is 14C-labelled, a low ratio 3H /14C stands for a tightly coupled reaction. The respecti ...
Can sugars be produced from fatty acids? A test
... In the 1950s, experiments using a new method involving isotopically labelled compounds started to reveal the mechanism by which carbons of fatty acids are incorporated in carbohydrates. Experiments showed that labelled carbons arrived at glucose when the system was supplied with 14 C-labelled fatty ...
... In the 1950s, experiments using a new method involving isotopically labelled compounds started to reveal the mechanism by which carbons of fatty acids are incorporated in carbohydrates. Experiments showed that labelled carbons arrived at glucose when the system was supplied with 14 C-labelled fatty ...
Biochemistry - Wikimedia Commons
... Biochemistry is the study of the chemistry of, and relating to, biological organisms. It forms a bridge between biology and chemistry by studying how complex chemical reactions and chemical structures give rise to life and life's processes. Biochemistry is sometimes viewed as a hybrid branch of orga ...
... Biochemistry is the study of the chemistry of, and relating to, biological organisms. It forms a bridge between biology and chemistry by studying how complex chemical reactions and chemical structures give rise to life and life's processes. Biochemistry is sometimes viewed as a hybrid branch of orga ...
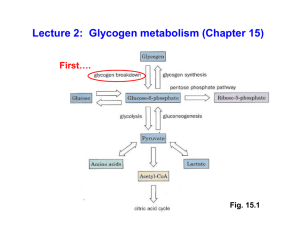
Regulation of carbohydrate metabolism
... 1. 3 key enzymes for the regulation of glycolysis (their activation). The role of Fructose 2,6-P in the regulation of glycolysis and gluconeogenesis. 2. 3 key sites for the regulation of gluconeogenesis (their activation). 3. The signal pathway for the activation of glycogen degradation by glucagon. ...
... 1. 3 key enzymes for the regulation of glycolysis (their activation). The role of Fructose 2,6-P in the regulation of glycolysis and gluconeogenesis. 2. 3 key sites for the regulation of gluconeogenesis (their activation). 3. The signal pathway for the activation of glycogen degradation by glucagon. ...
The phosphorylation of proteins: a major mechanism for biological
... activities. As to how many protein kinases exist, I think it is much too early t o tell, but some idea as t o their number and diversity can be seen in Table 1 and Table 2. Perhaps the best-characterized protein kinases are the family of cyclic AMP-dependent kinases. These exist in several isoenzymi ...
... activities. As to how many protein kinases exist, I think it is much too early t o tell, but some idea as t o their number and diversity can be seen in Table 1 and Table 2. Perhaps the best-characterized protein kinases are the family of cyclic AMP-dependent kinases. These exist in several isoenzymi ...
Proteins are made of chains of amino acids
... used for in the body. Describe the symptoms you would expect a person with protein deficiency to have. • Look at Table 1. Which amino acids does corn lack (not have)? Which amino acids do beans and legumes lack (not have)? • Vegans are vegetarians that do not eat any food from animals, including mil ...
... used for in the body. Describe the symptoms you would expect a person with protein deficiency to have. • Look at Table 1. Which amino acids does corn lack (not have)? Which amino acids do beans and legumes lack (not have)? • Vegans are vegetarians that do not eat any food from animals, including mil ...
Active site mapping, biochemical properties and
... spp. (leishmaniasis, [8]) have demonstrated potential as chemotherapeutic targets using peptidyl and peptidomimetic inhibitors [9]. In African trypanosomes, the major cysteine protease has a primary sequence and biochemical characteristics broadly similar to mammalian cathepsin L [10– 12]. Recently, ...
... spp. (leishmaniasis, [8]) have demonstrated potential as chemotherapeutic targets using peptidyl and peptidomimetic inhibitors [9]. In African trypanosomes, the major cysteine protease has a primary sequence and biochemical characteristics broadly similar to mammalian cathepsin L [10– 12]. Recently, ...
Antioxidant Activity Associated with Lipid and Phenolic Mobilization
... related to the four tocopherols associated with vitamin E, but tocotrienols are less widely distributed in nature. Tocopherols naturally present in foods have been strongly correlated with the polyunsaturated fatty acid because it counteracts the potential oxidative deterioration caused by fats in t ...
... related to the four tocopherols associated with vitamin E, but tocotrienols are less widely distributed in nature. Tocopherols naturally present in foods have been strongly correlated with the polyunsaturated fatty acid because it counteracts the potential oxidative deterioration caused by fats in t ...
Bacterial methionine biosynthesis
... other hydrophobic amino acids (valine, leucine and isoleucine). Additionally, S/p interactions between the side chain sulfur atom and aromatic amino acids have recently been identified as prevalent and important stabilizing interactions in one-third of all known protein structures (Valley et al., 20 ...
... other hydrophobic amino acids (valine, leucine and isoleucine). Additionally, S/p interactions between the side chain sulfur atom and aromatic amino acids have recently been identified as prevalent and important stabilizing interactions in one-third of all known protein structures (Valley et al., 20 ...
Problem Set 8 Key
... can be made from DHAP through a reduction of the C2 carbonyl to an alcohol . c. Determine how much ATP energy is sacrificed to make this lysophosphatidic acid. Make sure to account for the energy that could be made from the glucose that are consumed, any ATP that is directly consumed in the process, ...
... can be made from DHAP through a reduction of the C2 carbonyl to an alcohol . c. Determine how much ATP energy is sacrificed to make this lysophosphatidic acid. Make sure to account for the energy that could be made from the glucose that are consumed, any ATP that is directly consumed in the process, ...
22: Peptides, Proteins, and α
... peptide cleavage reactions uses the enzyme trypsin to cleave the peptide bond in CH(Rx)C(=O)—NHCH(Ry) where Rx is from Lys or Arg, while Ry is from any amino acid except Pro. As a result, the C-terminus of each cleaved segment is Lys or Arg, with the exception of the segment with the original C-term ...
... peptide cleavage reactions uses the enzyme trypsin to cleave the peptide bond in CH(Rx)C(=O)—NHCH(Ry) where Rx is from Lys or Arg, while Ry is from any amino acid except Pro. As a result, the C-terminus of each cleaved segment is Lys or Arg, with the exception of the segment with the original C-term ...
A modular approach to sphingolipid analogs mediated by aziridines: Synthesis
... Sphingolipid structures are defined by their eighteen carbon backbones with a 2‐amino‐1,3‐ diol functionality (usually 2S, 3R), which are called sphingoid bases. These organic bases can be N‐acylated by fatty acids of different length giving ceramides. Modification of this ge ...
... Sphingolipid structures are defined by their eighteen carbon backbones with a 2‐amino‐1,3‐ diol functionality (usually 2S, 3R), which are called sphingoid bases. These organic bases can be N‐acylated by fatty acids of different length giving ceramides. Modification of this ge ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.