Exam 2
... C. On the graph above, draw the double reciprocal curve you would expect if a transport inhibitor that was competitive with glucose binding were added. ...
... C. On the graph above, draw the double reciprocal curve you would expect if a transport inhibitor that was competitive with glucose binding were added. ...
Lecture exam 1A
... A. O2 serves as the final electron acceptor B. ethanol is a final product C. NADH is formed D. ATP is synthesized via oxidative phosphorylation E. many of the required enzymes are membrane-bound 35. The conversion of glyceraldehyde-3-phospate to 1,3-diphosphoglyceric acid is an important step in gly ...
... A. O2 serves as the final electron acceptor B. ethanol is a final product C. NADH is formed D. ATP is synthesized via oxidative phosphorylation E. many of the required enzymes are membrane-bound 35. The conversion of glyceraldehyde-3-phospate to 1,3-diphosphoglyceric acid is an important step in gly ...
Biochemistry - Bishop Ireton High School
... • In most chemical reactions this AE is in the form of heat • This heat energy moves the reactants called SUBSTRATES around causing them to bump into each other. • In the body, heat can’t be used as AE because it would harm the body. ...
... • In most chemical reactions this AE is in the form of heat • This heat energy moves the reactants called SUBSTRATES around causing them to bump into each other. • In the body, heat can’t be used as AE because it would harm the body. ...
Photosynthesis and Cellular Respiration
... What is Photosynthesis? • Using the sun’s energy to make food • Requires a pigment called chlorophyll • Occurs inside chloroplasts ...
... What is Photosynthesis? • Using the sun’s energy to make food • Requires a pigment called chlorophyll • Occurs inside chloroplasts ...
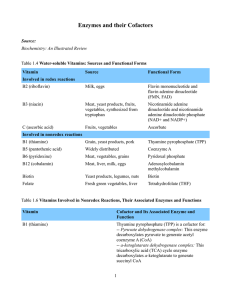
Enzymes and their Cofactors Source: Biochemistry: An Illustrated
... Pyridoxal phosphate is a cofactor for: -- Transaminases: These enzymes transfer amino groups between molecules to interconvert amino acids and alfa-keto acids -- Decarboxylases: These enzymes catalyze the removal of carboxyl groups. Specific decarboxylases play a critical role in the synthesis of ne ...
... Pyridoxal phosphate is a cofactor for: -- Transaminases: These enzymes transfer amino groups between molecules to interconvert amino acids and alfa-keto acids -- Decarboxylases: These enzymes catalyze the removal of carboxyl groups. Specific decarboxylases play a critical role in the synthesis of ne ...
Answer Key (up to 3/21)
... a. In ETC complex 1--NADH donates e- to FMN (Flavin-containing protein) which then donates to Fe•S (iron and sulfur-containing protein) and then passes electron to Q. b. In ETC complex 2--FADH2 donates electrons directly to Fe•S which then passes to Q. 12.) What is happening to the amount of potenti ...
... a. In ETC complex 1--NADH donates e- to FMN (Flavin-containing protein) which then donates to Fe•S (iron and sulfur-containing protein) and then passes electron to Q. b. In ETC complex 2--FADH2 donates electrons directly to Fe•S which then passes to Q. 12.) What is happening to the amount of potenti ...
Carbohydrate Metabolism: Glycolysis
... The initial materials can come directly from the chloroplast, from stored starch in an amyloplast, or from imported sucrose. The activation of fructose and glucose requires ATP. ...
... The initial materials can come directly from the chloroplast, from stored starch in an amyloplast, or from imported sucrose. The activation of fructose and glucose requires ATP. ...
How energy
... • High energy chemical intermediate (X) is formed during electron transfer from NADH to oxygen, then compound X transfer its energy to synthesis ATP. • You need to identify chemical nature of this putative intermediate. • This intermediate has never been found! • The Chemiosmotic Theory. ...
... • High energy chemical intermediate (X) is formed during electron transfer from NADH to oxygen, then compound X transfer its energy to synthesis ATP. • You need to identify chemical nature of this putative intermediate. • This intermediate has never been found! • The Chemiosmotic Theory. ...
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best
... 37) In an individual who is starving or fasting, the body meets its need for glucose first by the process of ________, and then by the process of ________. A) gluconeogenesis; glycogenesis B) lipogenesis; glycogenolysis C) glycogenolysis; gluconeogenesis D) glycogenesis; lipogenesis E) glycolysis; g ...
... 37) In an individual who is starving or fasting, the body meets its need for glucose first by the process of ________, and then by the process of ________. A) gluconeogenesis; glycogenesis B) lipogenesis; glycogenolysis C) glycogenolysis; gluconeogenesis D) glycogenesis; lipogenesis E) glycolysis; g ...
respiratory chain
... membranes. They inhibit phosphorylation because they decrease both electrical and pH gradient. The inner mitochondrial membrane is impermeable to most charged or hydrophilic substances. However it contains numerous transport proteins (carrier) that permit passage of specific molecules from the cytos ...
... membranes. They inhibit phosphorylation because they decrease both electrical and pH gradient. The inner mitochondrial membrane is impermeable to most charged or hydrophilic substances. However it contains numerous transport proteins (carrier) that permit passage of specific molecules from the cytos ...
Section 4. Overview of Fuel oxidation, ATP generation: Glycolysis is
... • Explain how NADH, FAD(2H) coenzymes carry electrons to electron transport chain • Describe how ATP synthesis is endergonic (requires energy) • Describe how ATP hydrolysis (exergonic) powers biosynthesis, movement, transport ...
... • Explain how NADH, FAD(2H) coenzymes carry electrons to electron transport chain • Describe how ATP synthesis is endergonic (requires energy) • Describe how ATP hydrolysis (exergonic) powers biosynthesis, movement, transport ...
Metabolism - UPM EduTrain Interactive Learning
... What happens to Pyruvate? Pyruvate is the final product of the 10 step pathway of glycolysis. The next step in the oxidation of glucose is the conversion of pyruvate to acetyl-CoA, and the subsequent oxidation of this two carbon compound to CO2 The metabolic pathway in which this occurs is a ...
... What happens to Pyruvate? Pyruvate is the final product of the 10 step pathway of glycolysis. The next step in the oxidation of glucose is the conversion of pyruvate to acetyl-CoA, and the subsequent oxidation of this two carbon compound to CO2 The metabolic pathway in which this occurs is a ...
Data/hora: 16/04/2017 16:28:45 Provedor de dados: 36 País: Brazil
... Palavras-chave: On-line pre-concentration; Inorganic anions; Organic anions; Ion chromatography; River waters. Resumo: An ion chromatography procedure, employing an IonPac AC15 concentrator column was used to investigate on line preconcentration for the simultaneous determination of inorganic anions ...
... Palavras-chave: On-line pre-concentration; Inorganic anions; Organic anions; Ion chromatography; River waters. Resumo: An ion chromatography procedure, employing an IonPac AC15 concentrator column was used to investigate on line preconcentration for the simultaneous determination of inorganic anions ...
Metabolism: Dissimilatory (energy, catabolic) metabolism
... Heterotrophy: dependent on organic substrates Distinction rarely absolute: ”autrotrophs” may require organic growth factors (vitamins). Usually refers to carbon source for assimilatory metabolism (autotrophs depend on C-1 compounds, that is CO2 or CH4). Photoautotrophs use light as energy source Che ...
... Heterotrophy: dependent on organic substrates Distinction rarely absolute: ”autrotrophs” may require organic growth factors (vitamins). Usually refers to carbon source for assimilatory metabolism (autotrophs depend on C-1 compounds, that is CO2 or CH4). Photoautotrophs use light as energy source Che ...
Chapter 4 - Cellular Metabolism
... Cellular respiration involves three interconnected series of reactions: glycolysis, the citric acid cycle, and the electron transport chain (Fig. 4.5). the end result of these reactions is that glucose is broken down to carbon dioxide and water and energy is released. Some of this energy is captured ...
... Cellular respiration involves three interconnected series of reactions: glycolysis, the citric acid cycle, and the electron transport chain (Fig. 4.5). the end result of these reactions is that glucose is broken down to carbon dioxide and water and energy is released. Some of this energy is captured ...
Jordan University of Science and Technology Faculty of Medicine
... Jordan University of Science and Technology Faculty of Medicine Department of Biochemistry and Molecular Biology ...
... Jordan University of Science and Technology Faculty of Medicine Department of Biochemistry and Molecular Biology ...
NVC Bio 120 lect 9 cell respiration
... must be converted to acetyl Coenzyme A (acetyl CoA), which links glycolysis to the citric acid cycle This step is carried out by a multienzyme complex that catalyses three reactions ...
... must be converted to acetyl Coenzyme A (acetyl CoA), which links glycolysis to the citric acid cycle This step is carried out by a multienzyme complex that catalyses three reactions ...
123 biochemistry - Jordan University of Science and Technology
... Jordan University of Science and Technology Faculty of Medicine Department of Biochemistry and Molecular Biology Biochemistry M123; Course Description and Objectives: This course deals with structure and properties of biomolecules, such as amino acids, proteins, carbohydrates, lipids, and nucleic ac ...
... Jordan University of Science and Technology Faculty of Medicine Department of Biochemistry and Molecular Biology Biochemistry M123; Course Description and Objectives: This course deals with structure and properties of biomolecules, such as amino acids, proteins, carbohydrates, lipids, and nucleic ac ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... Answer any two of the following, each within 1500 words; Draw diagrams wherever necessary: (2x20= 40 marks) 26. Elaborate on pH scale and pH meter. Add a note on Henderson Hasselbalch equation. 27. Write in detail about the steps involved in synthesis and degradation of fatty acids. 28. Analyze biom ...
... Answer any two of the following, each within 1500 words; Draw diagrams wherever necessary: (2x20= 40 marks) 26. Elaborate on pH scale and pH meter. Add a note on Henderson Hasselbalch equation. 27. Write in detail about the steps involved in synthesis and degradation of fatty acids. 28. Analyze biom ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.