Main Concepts Muscle structure, Oxidation of fats, Muscle types
... of energy storage in humans. 16. To utilise the stored energy of triacylglycerols they must first be broken down into their components, glycerol and fatty acids. 17. Fatty acids bind to albumin in the blood allowing them to be transported in a soluble form. These fatty acids are converted to activat ...
... of energy storage in humans. 16. To utilise the stored energy of triacylglycerols they must first be broken down into their components, glycerol and fatty acids. 17. Fatty acids bind to albumin in the blood allowing them to be transported in a soluble form. These fatty acids are converted to activat ...
1 - Wsfcs
... 28. How many proteins are there? _________________ 29. To link amino acids together, a _______ must be removed from the amino group and an _______ must be removed from the COOH to form water. The covalent bond is called a _________________ 30. The ______________________ and ______________________ of ...
... 28. How many proteins are there? _________________ 29. To link amino acids together, a _______ must be removed from the amino group and an _______ must be removed from the COOH to form water. The covalent bond is called a _________________ 30. The ______________________ and ______________________ of ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... oxidized states as they accept and donate electrons. ° Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. ° It then returns to its oxidized form as it passes electrons to its more electronegative ...
... oxidized states as they accept and donate electrons. ° Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. ° It then returns to its oxidized form as it passes electrons to its more electronegative ...
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
... oxidized states as they accept and donate electrons. Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. It then returns to its oxidized form as it passes electrons to its more electronegative ...
Chapter 18 Metabolic Pathways and Energy Production
... • NAD+ is produced and used to oxidize more glyceraldehyde3-phosphate (glycolysis), producing small amounts of ATP • increased amount of lactate causes muscles to become tired and sore After exercise, a person breathes heavily to repay the oxygen debt and reform pyruvate in the liver. ...
... • NAD+ is produced and used to oxidize more glyceraldehyde3-phosphate (glycolysis), producing small amounts of ATP • increased amount of lactate causes muscles to become tired and sore After exercise, a person breathes heavily to repay the oxygen debt and reform pyruvate in the liver. ...
Ch. 6 ppt
... • The path that electrons take on their way down from glucose to oxygen involves many steps. • The first step is an electron acceptor called NAD+. – The transfer of electrons from organic fuel to NAD+ reduces it to NADH. ...
... • The path that electrons take on their way down from glucose to oxygen involves many steps. • The first step is an electron acceptor called NAD+. – The transfer of electrons from organic fuel to NAD+ reduces it to NADH. ...
Chapter 14- RESPIRATION IN PLANTS Living cells require a
... ) and ADP is phosphorylated to ATP by the addition of Pi. Since oxidation of NADH and FADH2 is associated with the synthesis of ATP, it is called ‘Oxidative Phosphorylation’(Fig.14.3). Oxidation of one NADH and one FADH2 yields three and two ATP molecules respectively. The hydrogen atom (H+ ) and th ...
... ) and ADP is phosphorylated to ATP by the addition of Pi. Since oxidation of NADH and FADH2 is associated with the synthesis of ATP, it is called ‘Oxidative Phosphorylation’(Fig.14.3). Oxidation of one NADH and one FADH2 yields three and two ATP molecules respectively. The hydrogen atom (H+ ) and th ...
Free Fatty acids - Sheffield Metabolic Laboratory
... metabolites (IMs), include lactate, pyruvate, acetoacetate as well as 3-hydroxybutyrate and free fatty acids (or non-esterified, NEFA). All are normally present in blood and have a vital role in energy metabolism. These compounds are linked through a number of different pathways, which interact depe ...
... metabolites (IMs), include lactate, pyruvate, acetoacetate as well as 3-hydroxybutyrate and free fatty acids (or non-esterified, NEFA). All are normally present in blood and have a vital role in energy metabolism. These compounds are linked through a number of different pathways, which interact depe ...
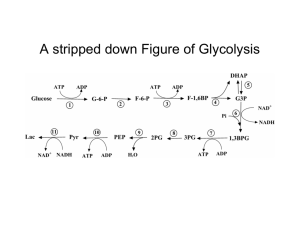
3 Physio Enzymes and Glycolysis
... Usually involves the transfer of 2H+ rather than free Remember…. electrons Electrons have to come from somewhere and go somewhere! ...
... Usually involves the transfer of 2H+ rather than free Remember…. electrons Electrons have to come from somewhere and go somewhere! ...
Microbiology - Chapter 7 & 8
... Anaerobes use a different set of enzymes, a Fermentative pathway that generates other acids, alcohols or gasses (lactic acid, ethanol, CO2) ** electron acceptor is an “organic molecule”** If no regeneration of NAD, the glycolysis pathway shuts down and the organism dies – no ATP ...
... Anaerobes use a different set of enzymes, a Fermentative pathway that generates other acids, alcohols or gasses (lactic acid, ethanol, CO2) ** electron acceptor is an “organic molecule”** If no regeneration of NAD, the glycolysis pathway shuts down and the organism dies – no ATP ...
userfiles/153/my files/09_lecture_presentation 2015?id=1069
... All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis BUT, they have different mechanisms for oxidizing NADH: In fermentation, an organic molecule (such as pyruvate or acetald ...
... All use glycolysis (net ATP = 2) to oxidize glucose and harvest chemical energy of food In all three, NAD+ is the oxidizing agent that accepts electrons during glycolysis BUT, they have different mechanisms for oxidizing NADH: In fermentation, an organic molecule (such as pyruvate or acetald ...
Biochem Molecules Presentation
... Proteins are synthesized by bonding amino acids Regents Biology ...
... Proteins are synthesized by bonding amino acids Regents Biology ...
The Water Cycle - Fall River Public Schools
... As warm moist air rises, it cools and forms clouds, then returns to the Earth as precipitation The rain seeps into the soil or enters a river or stream, and then runs off to the ocean ...
... As warm moist air rises, it cools and forms clouds, then returns to the Earth as precipitation The rain seeps into the soil or enters a river or stream, and then runs off to the ocean ...
03 - Respiration II, Photosynthesis I (ch.9,10) Sum13
... converted several steps, 4C lost (CO2) ...
... converted several steps, 4C lost (CO2) ...
Practice Exam #2.1 - Montana State University Billings
... E. The process of gas exchange across cell membranes 70. What properties of cell membranes prevent them from dissolving in water? A. The polar heads of the phospholipids B. Hydrogen bonding between the phosphate group of the phopholipids and water C. The hydrophobic hydrocarbon tails of the phosphol ...
... E. The process of gas exchange across cell membranes 70. What properties of cell membranes prevent them from dissolving in water? A. The polar heads of the phospholipids B. Hydrogen bonding between the phosphate group of the phopholipids and water C. The hydrophobic hydrocarbon tails of the phosphol ...
glucose-6-P - WordPress.com
... normal conditions, and so acts at a constant rate to provide glucose 6-phosphate to meet the cell's need. Liver cells also contain an isoenzyme of hexokinase, glucokinase, which has a Km very much higher than the normal intracellular concentration of glucose. The function of glucokinase in the liver ...
... normal conditions, and so acts at a constant rate to provide glucose 6-phosphate to meet the cell's need. Liver cells also contain an isoenzyme of hexokinase, glucokinase, which has a Km very much higher than the normal intracellular concentration of glucose. The function of glucokinase in the liver ...
CARBOHYDRATE METABOLISM - UNAIR | E
... blood 2. Gluconeogenesis 3. Glycogenolysis in liver • Insulin play a central role in regulating blood glucose blood glucose ...
... blood 2. Gluconeogenesis 3. Glycogenolysis in liver • Insulin play a central role in regulating blood glucose blood glucose ...
chapter 9 cellular respiration: harvesting chemical
... Redox reactions require both a donor and acceptor. Redox reactions also occur when the transfer of electrons is not complete but involves a change in the degree of electron sharing in covalent bonds. In the combustion of methane to form water and carbon dioxide, the nonpolar covalent bonds of ...
... Redox reactions require both a donor and acceptor. Redox reactions also occur when the transfer of electrons is not complete but involves a change in the degree of electron sharing in covalent bonds. In the combustion of methane to form water and carbon dioxide, the nonpolar covalent bonds of ...
Figure 17-3 Degradation of glucose via the glycolytic pathway.
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
cycles of matter worksheets
... 7. Plants and animals release CO2 into the atmosphere in a process called: respiration 8. How is carbon returned to the atmosphere? Organisms return carbon dioxide to the atmosphere by respiration. It is not just animals that respire. Plants, algae and microorganisms do too Carbon dioxide is also re ...
... 7. Plants and animals release CO2 into the atmosphere in a process called: respiration 8. How is carbon returned to the atmosphere? Organisms return carbon dioxide to the atmosphere by respiration. It is not just animals that respire. Plants, algae and microorganisms do too Carbon dioxide is also re ...
carbohydrate metabolism
... Acetyl-CoA is an ester of Coenzyme-A, which is the biologically active form of watersoluble vitamin Pantothenic acid TCA cycle occurs within Mitochondrial matrix under Aerobic condition Essentially TCA cycle comprises of combination of Acetyl-CoA with Oxaloacetate to give the Six-Carbon Tri-ca ...
... Acetyl-CoA is an ester of Coenzyme-A, which is the biologically active form of watersoluble vitamin Pantothenic acid TCA cycle occurs within Mitochondrial matrix under Aerobic condition Essentially TCA cycle comprises of combination of Acetyl-CoA with Oxaloacetate to give the Six-Carbon Tri-ca ...
A1983RT00700001
... utilis. Such was the elegance of their work catalyzed bya glutamate synthase enzyme that other people broadened their conclu- active with reduced ferredoxin (similar to sions to include the whole of the2 plant nitrite reductase) rather than reduced pyrikingdom. Although in 1969, Brown, work- dine nu ...
... utilis. Such was the elegance of their work catalyzed bya glutamate synthase enzyme that other people broadened their conclu- active with reduced ferredoxin (similar to sions to include the whole of the2 plant nitrite reductase) rather than reduced pyrikingdom. Although in 1969, Brown, work- dine nu ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.