Organic Chemistry/Fourth Edition: e-Text
... Acid-Base Properties of Amino Acids with Ionizable Side ...
... Acid-Base Properties of Amino Acids with Ionizable Side ...
Characterization and Surface Properties of Amino-Acid
... of different adsorption mechanisms that may be prevalent at different pHs. ...
... of different adsorption mechanisms that may be prevalent at different pHs. ...
Biochimica et Biophysica Acta (BBA) - Biomembranes 1768:
... assay, adapted from Pericon et al. [3] with minor modifications. Briefly, bacteria were grown in BHI medium at 37 °C under aerobic and anaerobic conditions until they reached OD620 = 0.2, centrifuged at 4 °C for 20 min at 4000×g, washed twice in ice cold phosphate buffered saline (PBS), pH = 7.4, an ...
... assay, adapted from Pericon et al. [3] with minor modifications. Briefly, bacteria were grown in BHI medium at 37 °C under aerobic and anaerobic conditions until they reached OD620 = 0.2, centrifuged at 4 °C for 20 min at 4000×g, washed twice in ice cold phosphate buffered saline (PBS), pH = 7.4, an ...
Crystal structure of potato tuber ADP‐glucose pyrophosphorylase
... Taken together, these studies indicate that the activator 3-PGA binds at or near the inhibitor-binding site defined in our structure by sulfate 1. Sulfate 2 makes interactions with surrounding residues, R53, R83, H84, Q314, and R316 (Figure 3A). All of these residues are conserved in both subunits of ...
... Taken together, these studies indicate that the activator 3-PGA binds at or near the inhibitor-binding site defined in our structure by sulfate 1. Sulfate 2 makes interactions with surrounding residues, R53, R83, H84, Q314, and R316 (Figure 3A). All of these residues are conserved in both subunits of ...
Document
... any permanent change or involved in the reaction. 35. What are ribozymes? Ans. Some nucleic acids behave like enzymes are called ribozymes. 36. What is the chemical nature of enzymes? Ans. Chemically enzymes are proteins. 37. What are the active sites of enzymes? Ans. An Active site of an enzyme is ...
... any permanent change or involved in the reaction. 35. What are ribozymes? Ans. Some nucleic acids behave like enzymes are called ribozymes. 36. What is the chemical nature of enzymes? Ans. Chemically enzymes are proteins. 37. What are the active sites of enzymes? Ans. An Active site of an enzyme is ...
Chlorine
... in the mercury cathode forming an amalgam. This flows continuously into a separate reactor ( " denuder " or " secondary cell " ) , where it is usually converted back to mercury by reaction with water, producing hydrogen and sodium ( or potassium ) hydroxide at a commercially useful concentration ( 5 ...
... in the mercury cathode forming an amalgam. This flows continuously into a separate reactor ( " denuder " or " secondary cell " ) , where it is usually converted back to mercury by reaction with water, producing hydrogen and sodium ( or potassium ) hydroxide at a commercially useful concentration ( 5 ...
Biochemistry2 2016 Lecture Glycogen Metabolism
... path on the right predominates when lactate is the precursor, because cytosolic NADH is generated in the lactate dehydrogenase reaction and does not have to be shuttled out of the mitochondrion. Constitutive to several pathways is pyruvate carboxylase which produces OAA. ...
... path on the right predominates when lactate is the precursor, because cytosolic NADH is generated in the lactate dehydrogenase reaction and does not have to be shuttled out of the mitochondrion. Constitutive to several pathways is pyruvate carboxylase which produces OAA. ...
Microbiology 146:
... reported. In comparison with the wild-type strain (CFN42), the GOGAT mutant strain utilized less succinate and glutamate and grew less with this and other amino acids as nitrogen source. R. etli assimilates ammonium by the glutamine synthetase (GS)-GOGAT pathway and a GOGAT mutant prevents the cycli ...
... reported. In comparison with the wild-type strain (CFN42), the GOGAT mutant strain utilized less succinate and glutamate and grew less with this and other amino acids as nitrogen source. R. etli assimilates ammonium by the glutamine synthetase (GS)-GOGAT pathway and a GOGAT mutant prevents the cycli ...
Enzymes and Vitamins Chapter 21 Problem
... 21.49 If each enzyme molecule is working to full capacity (saturated), a further increase in substrate concentration will have no effect on the rate of the reaction; the rate will remain constant. 21.50 number of substrate molecules transformed per molecule of enzyme per minute at optimum temperatur ...
... 21.49 If each enzyme molecule is working to full capacity (saturated), a further increase in substrate concentration will have no effect on the rate of the reaction; the rate will remain constant. 21.50 number of substrate molecules transformed per molecule of enzyme per minute at optimum temperatur ...
Enzymology Lectures Year 1 - Emily Flashman`s
... Most enzymes are selective about the chemical groups that will fit into their active sites Enzymes vary in their degree of specificity Most enzymes catalyse the reaction of a small range of related reactions e.g. yeast alcohol dehydrogenase (YADH) catalyses oxidation of small primary and secondary a ...
... Most enzymes are selective about the chemical groups that will fit into their active sites Enzymes vary in their degree of specificity Most enzymes catalyse the reaction of a small range of related reactions e.g. yeast alcohol dehydrogenase (YADH) catalyses oxidation of small primary and secondary a ...
cholesterol and lipo..
... Synthesis: About 50% of body cholesterol is derived from de novo biosynthesis. De novo synthesis of cholesterol occurs mainly in cytoplasm of liver ...
... Synthesis: About 50% of body cholesterol is derived from de novo biosynthesis. De novo synthesis of cholesterol occurs mainly in cytoplasm of liver ...
A novel assay method for an amino acid racemase reaction based
... The new assay method established in the present study for the measurement of the catalytic activity of ALR is based on the CD spectra of both enantiomers of Ala. The method is highly quantitative and provides visible data that reflect the exhaustive reaction of ALR. We conclude that the CD assay met ...
... The new assay method established in the present study for the measurement of the catalytic activity of ALR is based on the CD spectra of both enantiomers of Ala. The method is highly quantitative and provides visible data that reflect the exhaustive reaction of ALR. We conclude that the CD assay met ...
Print this article - PAGEPress Publications
... Oxalate is a toxic compound ubiquitous in the plant kingdom and widely consumed in normal human diets as a component of fruits, vegetables, grains and nuts (Hodgkinson, 1977). The normal daily intake of oxalate ranges from 70 to 920 mg, but strongly increases in vegetarians (Turroni et al., 2007). O ...
... Oxalate is a toxic compound ubiquitous in the plant kingdom and widely consumed in normal human diets as a component of fruits, vegetables, grains and nuts (Hodgkinson, 1977). The normal daily intake of oxalate ranges from 70 to 920 mg, but strongly increases in vegetarians (Turroni et al., 2007). O ...
5 organic chemistry: functional groups
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
... There is no change in the number of valence electrons on any of the atoms in the reaction. Both before and after the reaction, each carbon atom shares a total of eight valence electrons and each hydrogen atom shares two electrons. Instead of electrons, the reaction involves the transfer of atoms—in ...
СУМСЬКИЙ ДЕРЖАВНИЙ УНІВЕРСИТЕТ
... Review the definition of an acid (page 4). Hydrogen atoms in the acid molecules can be replaced by the metal atoms, as a result the salts are formed: Replacement of H atoms ...
... Review the definition of an acid (page 4). Hydrogen atoms in the acid molecules can be replaced by the metal atoms, as a result the salts are formed: Replacement of H atoms ...
University of Groningen Mutants and homologs of
... activating the hydroxyl group of Serβ1, which is the nucleophile in the first processing step [53-55] (Figure 3). If this water is forced out by mutating Pheβ177 to proline, the hydroxyl group is not positioned correctly and processing is inhibited [55]. In the nondeficient enzyme, the formed interm ...
... activating the hydroxyl group of Serβ1, which is the nucleophile in the first processing step [53-55] (Figure 3). If this water is forced out by mutating Pheβ177 to proline, the hydroxyl group is not positioned correctly and processing is inhibited [55]. In the nondeficient enzyme, the formed interm ...
enzyme
... compounds resembling the transition state of a catalyzed reaction should be very effective inhibitors of enzymes. These mimics are called transition state analogs. The transition state analogs use the hapten to generate antibodies with catalytic activity. These antibodies are called abzymes which ca ...
... compounds resembling the transition state of a catalyzed reaction should be very effective inhibitors of enzymes. These mimics are called transition state analogs. The transition state analogs use the hapten to generate antibodies with catalytic activity. These antibodies are called abzymes which ca ...
11 Cytochrome P450 and the Metabolism and Bioactivation of
... oxygen^ ^"^^. Moreover, while all these enzymes possess a heme-thiolate prosthetic group, their overall homology to other members of the P450 gene superfamily is limited and suggests an early evolutionary functional specialization^^"^^. The mechanism by which the hemoprotein cleaves the peroxide oxy ...
... oxygen^ ^"^^. Moreover, while all these enzymes possess a heme-thiolate prosthetic group, their overall homology to other members of the P450 gene superfamily is limited and suggests an early evolutionary functional specialization^^"^^. The mechanism by which the hemoprotein cleaves the peroxide oxy ...
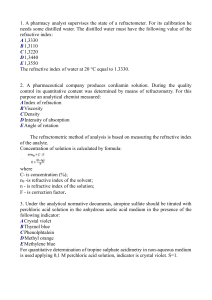
1. A pharmacy analyst supervises the state of a refractometer. For its
... 6. Diethyl ether relates to simple ethers. Prior to its identification by using the boiling temperature an analytical chemist must ensure that there are no: A Peroxides B Reducing substances C Alcohols D Non-volatile residue E Carboxylic acids Before determining the boiling temperature first determ ...
... 6. Diethyl ether relates to simple ethers. Prior to its identification by using the boiling temperature an analytical chemist must ensure that there are no: A Peroxides B Reducing substances C Alcohols D Non-volatile residue E Carboxylic acids Before determining the boiling temperature first determ ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.