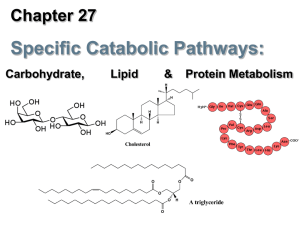
Lipid Metabolism 1. What has a higher stored energy potential per
... unhydrated as compared to glycogen which is very hydrated. 2. Acetyl CoA units are shuttled out of the mitochondria via citrate (which reacts with CoA in the cytosol to produce acetyl CoA and malate). The carbon units are returned to the mitochondria via pyruvate (following decarboxylation of malate ...
... unhydrated as compared to glycogen which is very hydrated. 2. Acetyl CoA units are shuttled out of the mitochondria via citrate (which reacts with CoA in the cytosol to produce acetyl CoA and malate). The carbon units are returned to the mitochondria via pyruvate (following decarboxylation of malate ...
i. building blocks
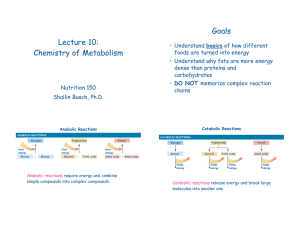
... II. SYNTHESIS AND DEGRADATION A. Dehydration synthesis 1. Monomers are joined to form polymers by the removal or a water molecule (dehydration) a) This results in covalent attachment of the subunits (1) The bond forms when a hydrogen from one monomer is linked to a hydroxyl group from another monome ...
... II. SYNTHESIS AND DEGRADATION A. Dehydration synthesis 1. Monomers are joined to form polymers by the removal or a water molecule (dehydration) a) This results in covalent attachment of the subunits (1) The bond forms when a hydrogen from one monomer is linked to a hydroxyl group from another monome ...
Secondary structure
... Peptidyl polymers • A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. • Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
... Peptidyl polymers • A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. • Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
C 4 plants
... yields energy – Used to pump H+ across the thylakoid membrane – Protons move from stroma into the thylakoid space • Flow of H+ back across the thylakoid membrane – Energizes ATP synthase, which – Enzymatically produces ATP from ADP + Pi • This method of producing ATP is called chemiosmosis • Photosy ...
... yields energy – Used to pump H+ across the thylakoid membrane – Protons move from stroma into the thylakoid space • Flow of H+ back across the thylakoid membrane – Energizes ATP synthase, which – Enzymatically produces ATP from ADP + Pi • This method of producing ATP is called chemiosmosis • Photosy ...
Lecture 10
... • ATP can also be made by the “electron transport chain”, the process by which the energy in free electrons is captured as ATP. Requires oxygen, makes water and carbon dioxide. ...
... • ATP can also be made by the “electron transport chain”, the process by which the energy in free electrons is captured as ATP. Requires oxygen, makes water and carbon dioxide. ...
Luminaries - Oxford Academic
... this muscle maintained its oxidative capacity after its disruption and suspension in aqueous media. In these studies, Dr. ...
... this muscle maintained its oxidative capacity after its disruption and suspension in aqueous media. In these studies, Dr. ...
Note - EtoosIndia
... The synthesis of energy rich ATP molecules with the help of energy librated by oxidation of reduced coenzyme produced during respiration is called Oxidative Phosphorylation. The protons which are expelled out from inner mitochondrial membrane during electron transport, produce proton gradient ( ...
... The synthesis of energy rich ATP molecules with the help of energy librated by oxidation of reduced coenzyme produced during respiration is called Oxidative Phosphorylation. The protons which are expelled out from inner mitochondrial membrane during electron transport, produce proton gradient ( ...
Production of L-4-phenyl-2-aminobutanoic acid by transamination
... transamination reactions using L-aspartic acid as the either thermally; chemically by various metal ions, amino donor regardless of the 2-keto acid precursor that amines and/or acids; or preferably enzymatically by the is used is the following: L-aspartic acid, a 2-keto acid, enzyme oxaloacetate dec ...
... transamination reactions using L-aspartic acid as the either thermally; chemically by various metal ions, amino donor regardless of the 2-keto acid precursor that amines and/or acids; or preferably enzymatically by the is used is the following: L-aspartic acid, a 2-keto acid, enzyme oxaloacetate dec ...
Document
... reduces all four ribonucleotides to their deoxyribo derivitives. A free radical mechanism is involved in the ribonucleotide reductase reaction. There are three classes of ribonucleotide reductase enzymes in nature: Class I: tyrosine radical, uses NDP Class II: adenosylcobalamin. uses NTPs (cyanobact ...
... reduces all four ribonucleotides to their deoxyribo derivitives. A free radical mechanism is involved in the ribonucleotide reductase reaction. There are three classes of ribonucleotide reductase enzymes in nature: Class I: tyrosine radical, uses NDP Class II: adenosylcobalamin. uses NTPs (cyanobact ...
Camp 1 - University of California, Santa Cruz
... H C OPO3 2 CH2 OH CH2 OPO3 22-Ph os phoglycerate ...
... H C OPO3 2 CH2 OH CH2 OPO3 22-Ph os phoglycerate ...
...the story of making proteins continued… After transcription occurs
... read the mRNA in 3’s. These 3letter chunks are called ___________________________. Each codon represents a specific _________________________________. Remember, amino acids make up ________________________! We are going to take this nucleotide message (mRNA) and use the coded dictionary to translat ...
... read the mRNA in 3’s. These 3letter chunks are called ___________________________. Each codon represents a specific _________________________________. Remember, amino acids make up ________________________! We are going to take this nucleotide message (mRNA) and use the coded dictionary to translat ...
Amino Acids : BCAA FLASH ZERO 360GR - BIOTECH
... internal organs, glands, hair and nails. They also contribute to producing hormones, as well as neurotransmitting substances and enzymes. Besides, they also serve as a source of energy, as well as a carbohydrate and lipid-producing nutrient. The richest source of basic amino acids are foods of anima ...
... internal organs, glands, hair and nails. They also contribute to producing hormones, as well as neurotransmitting substances and enzymes. Besides, they also serve as a source of energy, as well as a carbohydrate and lipid-producing nutrient. The richest source of basic amino acids are foods of anima ...
Metabolismus xenobiotik - Univerzita Karlova v Praze
... • hydrolyzis of its ester bond (intesine, blood) • conjugation in the liver with glycine → salicyluric acid • excretion of the conjugate with urine ...
... • hydrolyzis of its ester bond (intesine, blood) • conjugation in the liver with glycine → salicyluric acid • excretion of the conjugate with urine ...
Page 1 - csfcbiology
... (ii) This chemical test was carried out on samples A and B. All experimental variables were the same in the testing of the two samples. Both tubes were left for ten minutes to allow the precipitate to settle. The diagram shows the result. ...
... (ii) This chemical test was carried out on samples A and B. All experimental variables were the same in the testing of the two samples. Both tubes were left for ten minutes to allow the precipitate to settle. The diagram shows the result. ...
introacidbase
... – What is a protein’s structure and what role does it play in the body? – What are some important proteins in the body? – What are some key principles behind protein’s functions? ...
... – What is a protein’s structure and what role does it play in the body? – What are some important proteins in the body? – What are some key principles behind protein’s functions? ...
Chem 322 - Exam #4 - Spring 2003 - Answers
... stereoisomeric forms of this compound in the laboratory. The isomers are diastereomers. (d) This compound is achiral. At room temperature tetrahedral nitrogen rapidly inverts its configuration – the unshared pair of electrons passes through the nitrogen and comes out the other side, then repeats the ...
... stereoisomeric forms of this compound in the laboratory. The isomers are diastereomers. (d) This compound is achiral. At room temperature tetrahedral nitrogen rapidly inverts its configuration – the unshared pair of electrons passes through the nitrogen and comes out the other side, then repeats the ...
Glycolysis - Oregon State University
... The aldolase reaction puts together pieces so A fructose molecule is made with two phosphates in tow Metabolic Melody gluconeogenesis liver’s specialty And one of Oh these gets cleaved offis by a fructose phosphatase Producing sugar foracting the body most admirably (slow) Unless F2,6BP's blocking p ...
... The aldolase reaction puts together pieces so A fructose molecule is made with two phosphates in tow Metabolic Melody gluconeogenesis liver’s specialty And one of Oh these gets cleaved offis by a fructose phosphatase Producing sugar foracting the body most admirably (slow) Unless F2,6BP's blocking p ...
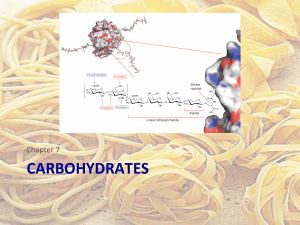
carbohydrates
... Monosaccharide derivatives- glycosides • Glycoside: a carbohydrate in which the -‐OH of the anomeric carbon is replaced by -‐OR • those derived from furanoses are furanosides; those derived from pyranoses are pyran ...
... Monosaccharide derivatives- glycosides • Glycoside: a carbohydrate in which the -‐OH of the anomeric carbon is replaced by -‐OR • those derived from furanoses are furanosides; those derived from pyranoses are pyran ...
10.25-11.3.11 Glycolysis
... •Cells are far away from equilibrium and far away from standard state conditions. We have much more ATP than would be dictated by equilibrium; the ratio of ATP to ADP+Pi in some cells is as high as 200/1 rather than 1/200,000. •This means that a cell can be far from equilibrium w.r. to this ratio, a ...
... •Cells are far away from equilibrium and far away from standard state conditions. We have much more ATP than would be dictated by equilibrium; the ratio of ATP to ADP+Pi in some cells is as high as 200/1 rather than 1/200,000. •This means that a cell can be far from equilibrium w.r. to this ratio, a ...
NYOS Charter School
... 13. Recall that heat flow out of a system at constant pressure is equal to the enthalpy change H and can be calculated by the equation shown below when using a coffee cup calorimeter. Given an initial temperature of 23.2 C of 55 grams of a water in the calorimeter (whose specific heat Cs is 4.184 ...
... 13. Recall that heat flow out of a system at constant pressure is equal to the enthalpy change H and can be calculated by the equation shown below when using a coffee cup calorimeter. Given an initial temperature of 23.2 C of 55 grams of a water in the calorimeter (whose specific heat Cs is 4.184 ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.