09.08.11 Chemistry of Amino Acids
... • Isoelectric Precipitation: Amino acids with net charges repel each other and do not easily crystallize from solution - solubility is lowest at the isoelectric point • CaM, protein that binds to Ca+2 ions, pI= 3, this protein has many many negative side groups such as glu and asp. At neutral pH, ...
... • Isoelectric Precipitation: Amino acids with net charges repel each other and do not easily crystallize from solution - solubility is lowest at the isoelectric point • CaM, protein that binds to Ca+2 ions, pI= 3, this protein has many many negative side groups such as glu and asp. At neutral pH, ...
8/27/08 Transcript I
... acid, which in never found inside cells, because it is furnished into another fatty acid and then others, finally into C16 which will diffuse ...
... acid, which in never found inside cells, because it is furnished into another fatty acid and then others, finally into C16 which will diffuse ...
1. phylum: firmicutes - Fermentation-SN
... Clostridium tetani is the causative agent of tetanus. The mycoplasmas, or Mollicutes, lack a cell wall, but are close relatives of Clostridium—many species, such as Mycoplasma pneumoniae, are pathogenic to humans. Micrococcus spp. differ from Staphylococcus in that they are obligate aerobes that are ...
... Clostridium tetani is the causative agent of tetanus. The mycoplasmas, or Mollicutes, lack a cell wall, but are close relatives of Clostridium—many species, such as Mycoplasma pneumoniae, are pathogenic to humans. Micrococcus spp. differ from Staphylococcus in that they are obligate aerobes that are ...
Document
... AMP or GMP is metabolized to give hypoxanthine which is then converted into xanthine and finally into uric acid as in the next slide. Most of uric acid is excreted by the kidney. The remaining uric acid travels through the intestines, where bacteria help break it down. Normally these actions keep th ...
... AMP or GMP is metabolized to give hypoxanthine which is then converted into xanthine and finally into uric acid as in the next slide. Most of uric acid is excreted by the kidney. The remaining uric acid travels through the intestines, where bacteria help break it down. Normally these actions keep th ...
Mary Jones Jennifer Gregory - Assets
... 2 describe the structure of ATP as a phosphorylated nucleotide; 3 describe the universal role of ATP as the energy ‘currency’ in living organisms; 4 explain that the synthesis of ATP is associated with the electron transport chain on the membranes of the mitochondrion; 5 outline glycolysis as the ph ...
... 2 describe the structure of ATP as a phosphorylated nucleotide; 3 describe the universal role of ATP as the energy ‘currency’ in living organisms; 4 explain that the synthesis of ATP is associated with the electron transport chain on the membranes of the mitochondrion; 5 outline glycolysis as the ph ...
Amino Acids
... Amino acids are the building blocks of proteins; proteins are made of amino acids. When you ingest a protein your body breaks it down into the individual aminos, reorders them, re-folds them, and turns them into whatever is needed by the body at that time. From only 20 amino acids, the body is able ...
... Amino acids are the building blocks of proteins; proteins are made of amino acids. When you ingest a protein your body breaks it down into the individual aminos, reorders them, re-folds them, and turns them into whatever is needed by the body at that time. From only 20 amino acids, the body is able ...
the code of translation
... • An Anticodon is the complimentary group of three nucleotides on a tRNA • When the codon is recognized by the complimentary anticodon on the tRNA the correct amino acid corresponding to that codon is made available. ...
... • An Anticodon is the complimentary group of three nucleotides on a tRNA • When the codon is recognized by the complimentary anticodon on the tRNA the correct amino acid corresponding to that codon is made available. ...
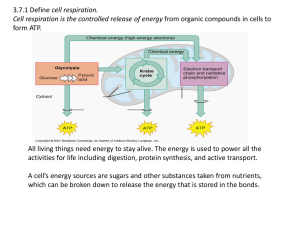
3.7:Cell Respiration Aerobic cell respiration: glucose
... The next stage of cell respiration depends on whether there is oxygen present or not. ...
... The next stage of cell respiration depends on whether there is oxygen present or not. ...
... in the presence of NADH or NADPH,7 while a cell-free particulate preparation of Penicillium patulum catalyzed the epoxidation of gentisyl alcohol (a hydroquinone) in the absence of any added cofactor,24as we now report for two Streptomyces enzymes. Consistent with the mechanism proposed for the P. p ...
lipid
... in many tissues by regulating the synthesis of the intracellular messenger 3_,5_-cyclic AMP (cAMP). Because cAMP mediates the action of diverse hormones, the prostaglandins affect a wide range of cellular and tissue functions. Some prostaglandins stimulate contraction of the smooth muscle of the ute ...
... in many tissues by regulating the synthesis of the intracellular messenger 3_,5_-cyclic AMP (cAMP). Because cAMP mediates the action of diverse hormones, the prostaglandins affect a wide range of cellular and tissue functions. Some prostaglandins stimulate contraction of the smooth muscle of the ute ...
Document
... 2) Coenzymes 3) Glycolysis 4) Lactate A. 4 Produced during anaerobic conditions. B. 3 Reaction series that converts glucose to pyruvate. C. 1 Metabolic reactions that break down large molecules to smaller molecules + energy. D. 2 Substances that remove or add H atoms in oxidation and reduction react ...
... 2) Coenzymes 3) Glycolysis 4) Lactate A. 4 Produced during anaerobic conditions. B. 3 Reaction series that converts glucose to pyruvate. C. 1 Metabolic reactions that break down large molecules to smaller molecules + energy. D. 2 Substances that remove or add H atoms in oxidation and reduction react ...
Impact of Ischemia on Cellular Metabolism
... 2. Adenosine triphosphate depletion Eukaryotic cells contain mitochondria, organelles whose main function is to produce adeno‐ sine triphosphate (ATP). ATP is an essential energy substrate, as its hydrolysis provides en‐ ergy for many metabolic and biochemical reactions involved in development, adap ...
... 2. Adenosine triphosphate depletion Eukaryotic cells contain mitochondria, organelles whose main function is to produce adeno‐ sine triphosphate (ATP). ATP is an essential energy substrate, as its hydrolysis provides en‐ ergy for many metabolic and biochemical reactions involved in development, adap ...
pentose phosphate pathway
... 2. Fructose-1,6-bisphosphatase replaces the phosphofructokinase reaction of glycolysis. 3. Glucose-6-phosphatase replaces the hexokinase reaction of glycolysis. • The new reactions provide for a spontaneous pathway (DG negative in the direction of sugar synthesis), and they provide new mechanisms of ...
... 2. Fructose-1,6-bisphosphatase replaces the phosphofructokinase reaction of glycolysis. 3. Glucose-6-phosphatase replaces the hexokinase reaction of glycolysis. • The new reactions provide for a spontaneous pathway (DG negative in the direction of sugar synthesis), and they provide new mechanisms of ...
biochemistry national board exam review
... reaction, which of the following would be true about that enzyme? A. C would be a competitive inhibitor of the enzyme. B. C would be a noncompetitive inhibitor of the enzyme. C. The velocity vs. [S] plot for the enzyme would be the same with or without C. D. With C present, the enzyme would convert ...
... reaction, which of the following would be true about that enzyme? A. C would be a competitive inhibitor of the enzyme. B. C would be a noncompetitive inhibitor of the enzyme. C. The velocity vs. [S] plot for the enzyme would be the same with or without C. D. With C present, the enzyme would convert ...
Valine Mydrogenase from Streptmzyces fiadipe
... After SDS-PAGE the enzyme exhibited a single band correspondingto an M,of about 18000. Thus, the enzyme appears to consist of 12 subunits of similar M,. Isoelectric focusing of the VDH in a polyacrylamidegel showed the PI value to be 4.7 (Fig. 1). Eflect of p H and temperature on the erczynte activi ...
... After SDS-PAGE the enzyme exhibited a single band correspondingto an M,of about 18000. Thus, the enzyme appears to consist of 12 subunits of similar M,. Isoelectric focusing of the VDH in a polyacrylamidegel showed the PI value to be 4.7 (Fig. 1). Eflect of p H and temperature on the erczynte activi ...
BIOCHEMISTRY NATIONAL BOARD EXAM REVIEW
... reaction, which of the following would be true about that enzyme? A. C would be a competitive inhibitor of the enzyme. B. C would be a noncompetitive inhibitor of the enzyme. C. The velocity vs. [S] plot for the enzyme would be the same with or without C. D. With C present, the enzyme would convert ...
... reaction, which of the following would be true about that enzyme? A. C would be a competitive inhibitor of the enzyme. B. C would be a noncompetitive inhibitor of the enzyme. C. The velocity vs. [S] plot for the enzyme would be the same with or without C. D. With C present, the enzyme would convert ...
File - Pre
... • Steroids are lipids that are made from carbon rings • Steroids have important functions in living things – A) Cholesterol: a steroid that helps keep cell membranes in animal cells structurally sound – B) Steroid hormones: steroids that help control biological reactions ...
... • Steroids are lipids that are made from carbon rings • Steroids have important functions in living things – A) Cholesterol: a steroid that helps keep cell membranes in animal cells structurally sound – B) Steroid hormones: steroids that help control biological reactions ...
AS Biology - TavistockCollegeScience
... Cellulose makes up plant cell walls It is a structural polysaccharide It is made up of β glucose where OH is above the ring In order to form a glycosidic bond the other glucose must be upside down. The bond formed is a β1-4 glycosidic bond ...
... Cellulose makes up plant cell walls It is a structural polysaccharide It is made up of β glucose where OH is above the ring In order to form a glycosidic bond the other glucose must be upside down. The bond formed is a β1-4 glycosidic bond ...
BCAA 4:1:1 - ProAction
... BCAA are metabolized in the mitochondria; valine is converted into a molecule of succinyl-CoA, a Krebs cycle intermediate; isoleucine generates one molecule of succinyl-CoA and one of acetyl-CoA; and the complete catabolism of leucine produces three molecules of acetyl-CoA, and this process continue ...
... BCAA are metabolized in the mitochondria; valine is converted into a molecule of succinyl-CoA, a Krebs cycle intermediate; isoleucine generates one molecule of succinyl-CoA and one of acetyl-CoA; and the complete catabolism of leucine produces three molecules of acetyl-CoA, and this process continue ...
Determination of Pyruvate Oxidation Rate and Citric Acid Cycle
... of ATP quantitatively depends less on oxidative phosphorylation than on glycolysis ...
... of ATP quantitatively depends less on oxidative phosphorylation than on glycolysis ...
Aminoacids_followup
... Amino acids with hydroxyl group In biology hydroxyl groups –OH are important as they can be modified by different molecules as phosphate (-PO4) or a long range of ...
... Amino acids with hydroxyl group In biology hydroxyl groups –OH are important as they can be modified by different molecules as phosphate (-PO4) or a long range of ...
1 The diagram below represents a biological process 5
... 18. What is the chemical compound represented by letter J? 1) a protease 3) ATP 2) a polysaccharide 4) ADP 19. Lipase, maltase, and protease are members of a group of catalysts known as 1) enzymes 3) carbohydrates 2) hormones 4) fats 20. Hydrogen peroxide (H2O2) is a toxic by-product of cellular me ...
... 18. What is the chemical compound represented by letter J? 1) a protease 3) ATP 2) a polysaccharide 4) ADP 19. Lipase, maltase, and protease are members of a group of catalysts known as 1) enzymes 3) carbohydrates 2) hormones 4) fats 20. Hydrogen peroxide (H2O2) is a toxic by-product of cellular me ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.