sample information form”.
... Tel.: e-mail: 1- Information on molecule(s) to be analysed 1.1 - Information on molecule n°1 (duplicate, if more than one): Name (or acronym, if confidential): Abbreviation: Chemical nature (protein, …) and origin of molecule (organism, …): Sequence or chemical formula: (for proteins, include tags w ...
... Tel.: e-mail: 1- Information on molecule(s) to be analysed 1.1 - Information on molecule n°1 (duplicate, if more than one): Name (or acronym, if confidential): Abbreviation: Chemical nature (protein, …) and origin of molecule (organism, …): Sequence or chemical formula: (for proteins, include tags w ...
Bioinformatics Needs for the post
... Protein structure prediction an example • There are over 1.3 million sequences in the nonredundant protein database managed by the NCBI and over 19 thousand structures in the protein data bank (PDB) • Using this data we have built a library of common protein substructures linking structure and sequ ...
... Protein structure prediction an example • There are over 1.3 million sequences in the nonredundant protein database managed by the NCBI and over 19 thousand structures in the protein data bank (PDB) • Using this data we have built a library of common protein substructures linking structure and sequ ...
Cardiff International School Dhaka (CISD) Lost Class Make Up
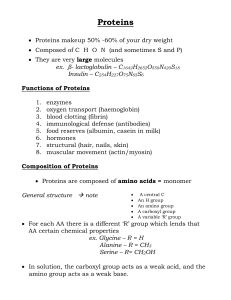
... Q1. a) The primary structure of a protein is the level of protein structure which refers to the specific sequence of amino acids. When two amino acids are in such a position that the carboxyl groups of each amino acid are adjacent to each other, they can be combined by undergoing a dehydration react ...
... Q1. a) The primary structure of a protein is the level of protein structure which refers to the specific sequence of amino acids. When two amino acids are in such a position that the carboxyl groups of each amino acid are adjacent to each other, they can be combined by undergoing a dehydration react ...
Huang, David, Center for Structural Biochemistry
... Crystallization – Purified proteins were prepared with various solvent and ligands on crystallography plates. Crystals were collected after a few days. ...
... Crystallization – Purified proteins were prepared with various solvent and ligands on crystallography plates. Crystals were collected after a few days. ...
Self Test Quiz-1 Given below are some questions related to protein
... Given below are some questions related to protein and enzymes in general. Each statement is followed by 4 choices. Choose a single correct answer for each question. 1. How many different types of amino acid are used to make proteins? a. 4 b. 20 c. 23 d. 38 2. Amino acids contain carbon, hydrogen, ox ...
... Given below are some questions related to protein and enzymes in general. Each statement is followed by 4 choices. Choose a single correct answer for each question. 1. How many different types of amino acid are used to make proteins? a. 4 b. 20 c. 23 d. 38 2. Amino acids contain carbon, hydrogen, ox ...
NMR Stucture of the Sterol Carrier Protein
... Present address: Dr Thomas Szyperski, Department of Chemistry, 816 Natural Sciences Complex, State University of New York at Buffalo, Buffalo, NY 14260, USA. Abbreviations used: SCP2, sterol carrier protein-2; SCPX, sterol carrier protein-X; hSCP2, human SCP2 with residues 1-123; rSCP2, rabbit SCP2; ...
... Present address: Dr Thomas Szyperski, Department of Chemistry, 816 Natural Sciences Complex, State University of New York at Buffalo, Buffalo, NY 14260, USA. Abbreviations used: SCP2, sterol carrier protein-2; SCPX, sterol carrier protein-X; hSCP2, human SCP2 with residues 1-123; rSCP2, rabbit SCP2; ...
Document
... intrinsic magnetic moment. The most commonly used nuclei are hydrogen-1 and carbon-13, although certain isotopes of many other elements nuclei can also be observed. NMR studies a magnetic nucleus, like that of a hydrogen atom (protium being the most receptive isotope at natural abundance) by alignin ...
... intrinsic magnetic moment. The most commonly used nuclei are hydrogen-1 and carbon-13, although certain isotopes of many other elements nuclei can also be observed. NMR studies a magnetic nucleus, like that of a hydrogen atom (protium being the most receptive isotope at natural abundance) by alignin ...
Ultrafast Solvation: Investigating Molecular Forces in Protein Folding November 12, 2010
... investigate solvent-protein interactions by measuring the photoexcitation of the natural amino acid tryptophan using ultrafast fluorescence spectroscopy. This allows us to probe the solvent dynamics at the surface of the molecule. Using this technique we investigated how the variation of solvent pro ...
... investigate solvent-protein interactions by measuring the photoexcitation of the natural amino acid tryptophan using ultrafast fluorescence spectroscopy. This allows us to probe the solvent dynamics at the surface of the molecule. Using this technique we investigated how the variation of solvent pro ...
Proteomics techniques used to identify proteins
... Proteomics techniques used to identify proteins ...
... Proteomics techniques used to identify proteins ...
Protein: How Cows and Carrots Become People 1. Your body can
... Protein: How Cows and Carrots Become People 1. Your body can manufacture some amino acids (used as the building blocks of proteins) out of nitrogen. What name is given to this group of amino acids? ...
... Protein: How Cows and Carrots Become People 1. Your body can manufacture some amino acids (used as the building blocks of proteins) out of nitrogen. What name is given to this group of amino acids? ...
Rational Drug Design
... Good targets for drug design Drug Zanamavir (marketed as Relenza) binds to the neuraminidase protein (N) ...
... Good targets for drug design Drug Zanamavir (marketed as Relenza) binds to the neuraminidase protein (N) ...
Proteins - Wesleyan College Faculty
... Proteins • Primary Structure – Sequence of Amino Acids – Met-Gly-Ser-His-Ala- Leu-Trp-Ile-Thr ...
... Proteins • Primary Structure – Sequence of Amino Acids – Met-Gly-Ser-His-Ala- Leu-Trp-Ile-Thr ...
Key Points Folding
... Key Points Prions and Protein Folding • Protein structure (primary, secondary, tertiary) • Proteins have many possible conformations (ways to fold up into a 3D structure) • Proteins can spontaneously fold into the correct (biologically functional) 3D structure demonstrated by Christian Anfinsen in t ...
... Key Points Prions and Protein Folding • Protein structure (primary, secondary, tertiary) • Proteins have many possible conformations (ways to fold up into a 3D structure) • Proteins can spontaneously fold into the correct (biologically functional) 3D structure demonstrated by Christian Anfinsen in t ...
Nuclear magnetic resonance spectroscopy of proteins
Nuclear magnetic resonance spectroscopy of proteins (usually abbreviated protein NMR) is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins, and also nucleic acids, and their complexes. The field was pioneered by Richard R. Ernst and Kurt Wüthrich at the ETH, and by Ad Bax, Marius Clore and Angela Gronenborn at the NIH, among others. Structure determination by NMR spectroscopy usually consists of several phases, each using a separate set of highly specialized techniques. The sample is prepared, measurements are made, interpretive approaches are applied, and a structure is calculated and validated.NMR involves the quantum mechanical properties of the central core (""nucleus"") of the atom. These properties depend on the local molecular environment, and their measurement provides a map of how the atoms are linked chemically, how close they are in space, and how rapidly they move with respect to each other. These properties are fundamentally the same as those used in the more familiar Magnetic Resonance Imaging (MRI), but the molecular applications use a somewhat different approach, appropriate to the change of scale from millimeters (of interest to radiologists) to nano-meters (bonded atoms are typically a fraction of a nano-meter apart), a factor of a million. This change of scale requires much higher sensitivity of detection and stability for long term measurement. In contrast to MRI, structural biology studies do not directly generate an image, but rely on complex computer calculations to generate three-dimensional molecular models.Currently most samples are examined in a solution in water, but methods are being developed to also work with solid samples. Data collection relies on placing the sample inside a powerful magnet, sending radio frequency signals through the sample, and measuring the absorption of those signals. Depending on the environment of atoms within the protein, the nuclei of individual atoms will absorb different frequencies of radio signals. Furthermore the absorption signals of different nuclei may be perturbed by adjacent nuclei. This information can be used to determine the distance between nuclei. These distances in turn can be used to determine the overall structure of the protein.A typical study might involve how two proteins interact with each other, possibly with a view to developing small molecules that can be used to probe the normal biology of the interaction (""chemical biology"") or to provide possible leads for pharmaceutical use (drug development). Frequently, the interacting pair of proteins may have been identified by studies of human genetics, indicating the interaction can be disrupted by unfavorable mutations, or they may play a key role in the normal biology of a ""model"" organism like the fruit fly, yeast, the worm C. elegans, or mice. To prepare a sample, methods of molecular biology are typically used to make quantities by bacterial fermentation. This also permits changing the isotopic composition of the molecule, which is desirable because the isotopes behave differently and provide methods for identifying overlapping NMR signals.