Populations and their Ecosystems
... depend upon plants for sulfur. Plants absorb sulfur when it is dissolved in water. Animals consume these plants, so that they take up enough sulfur to maintain their health. Most of the earth's sulfur is tied up in rocks and salts or buried deep in the ocean in oceanic sediments. Sulfur can also be ...
... depend upon plants for sulfur. Plants absorb sulfur when it is dissolved in water. Animals consume these plants, so that they take up enough sulfur to maintain their health. Most of the earth's sulfur is tied up in rocks and salts or buried deep in the ocean in oceanic sediments. Sulfur can also be ...
III. Cells and Energy
... (from the atmosphere) and hydrogen (from NADPH) to form glucose in a series of reactions called the Calvin Cycle ...
... (from the atmosphere) and hydrogen (from NADPH) to form glucose in a series of reactions called the Calvin Cycle ...
Chapter 9: The Need for Energy
... The process by which mitochondria break down glucose to make ATP Two types o Aerobic respiration: requires oxygen and carried out by plants, animals, and some bacteria o Anaerobic respiration: requires no oxygen and carried out by yeast, some bacteria, and sometimes animals Chemical equation for ...
... The process by which mitochondria break down glucose to make ATP Two types o Aerobic respiration: requires oxygen and carried out by plants, animals, and some bacteria o Anaerobic respiration: requires no oxygen and carried out by yeast, some bacteria, and sometimes animals Chemical equation for ...
Nitrogen Cycle Presenter: ___ Nitrogen Fixation: ___ Atmosphere
... ___ Ocean water – absorbs and stores carbon dioxide ___ Causes water to become acidic ___ forms Carbonic acid, HCO3 ___ Carbonate, CO23- or bicarbonate ions, HCO3- are released into atmosphere as sun’s energy converts them ___ Calcium Carbonate, CaCO3 in water is used by corals and oysters for shell ...
... ___ Ocean water – absorbs and stores carbon dioxide ___ Causes water to become acidic ___ forms Carbonic acid, HCO3 ___ Carbonate, CO23- or bicarbonate ions, HCO3- are released into atmosphere as sun’s energy converts them ___ Calcium Carbonate, CaCO3 in water is used by corals and oysters for shell ...
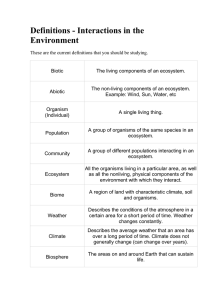
Definitions - Interactions in the Environment These are the current
... All the organisms living in a particular area, as well as all the nonliving, physical components of the environment with which they interact. ...
... All the organisms living in a particular area, as well as all the nonliving, physical components of the environment with which they interact. ...
Ecology Unit
... Nitrogen cycleOnly in certain bacteria and industrial technologies can fix nitrogen. Nitrogen fixation-convert atmospheric nitrogen (N2) into ammonium (NH4+) which can be used to make organic compounds like amino acids. ...
... Nitrogen cycleOnly in certain bacteria and industrial technologies can fix nitrogen. Nitrogen fixation-convert atmospheric nitrogen (N2) into ammonium (NH4+) which can be used to make organic compounds like amino acids. ...
Understanding Our Environment
... photosynthesis. Carbon atoms are incorporated into glucose and then: Remain in plant material until death. - Eaten by predator ...
... photosynthesis. Carbon atoms are incorporated into glucose and then: Remain in plant material until death. - Eaten by predator ...
Review Guide Answer Key
... What is the difference between a food chain and food web? Food chain shows only one feeding relationships, a food web shows multiple feeding relationships and is more realistic The original source of energy for all living organisms is the _SUN!!!!_. Each step in a food web is called a __Trophic Leve ...
... What is the difference between a food chain and food web? Food chain shows only one feeding relationships, a food web shows multiple feeding relationships and is more realistic The original source of energy for all living organisms is the _SUN!!!!_. Each step in a food web is called a __Trophic Leve ...
History of the Earth - Green Local Schools
... no fossils, then came simple sea creatures, then more complex ones like fishes, then came life on land, then reptiles, then mammals, and finally humans. Clearly, there was some kind of 'progress' going on. ...
... no fossils, then came simple sea creatures, then more complex ones like fishes, then came life on land, then reptiles, then mammals, and finally humans. Clearly, there was some kind of 'progress' going on. ...
PowerPoint Presentation - Ch. 6 Cellular Respiration
... • When ATP is made by movement of Hydrogen ions from high to low concentration via the protein ATP synthase. • How does a high concentration of hydrogen ions form in the first place? • H+ ions are actively transported using electron energy ...
... • When ATP is made by movement of Hydrogen ions from high to low concentration via the protein ATP synthase. • How does a high concentration of hydrogen ions form in the first place? • H+ ions are actively transported using electron energy ...
Original
... - Interdependence (Interconnectedness): the dependence of every organism on its connections with other living and nonliving parts of its environment - Interdependence is a key theme found throughout ecology o Ex. You could not survive without the plants and other photosynthetic organisms that produc ...
... - Interdependence (Interconnectedness): the dependence of every organism on its connections with other living and nonliving parts of its environment - Interdependence is a key theme found throughout ecology o Ex. You could not survive without the plants and other photosynthetic organisms that produc ...
Name
... CO2= (1 pyruvate dehydrogenase + 2 TCA) = 3 CO2 Total ATP Produced following electron transport by all of the above mitochondrial reactions:___1+12+2=15ATP 2) Draw a diagram that shows with names or numbers the specific enzymes and pathways that feed electrons from FADH2 into electron transport and ...
... CO2= (1 pyruvate dehydrogenase + 2 TCA) = 3 CO2 Total ATP Produced following electron transport by all of the above mitochondrial reactions:___1+12+2=15ATP 2) Draw a diagram that shows with names or numbers the specific enzymes and pathways that feed electrons from FADH2 into electron transport and ...
The ATP-PCr energy system can operate with or without oxygen but
... The aerobic system, which is dependent on oxygen, is the most complex of the three energy systems. The metabolic reactions that take place in the presence of oxygen are responsible for most of the cellular energy produced by the body. However, aerobic metabolism is the slowest way to resynthesize AT ...
... The aerobic system, which is dependent on oxygen, is the most complex of the three energy systems. The metabolic reactions that take place in the presence of oxygen are responsible for most of the cellular energy produced by the body. However, aerobic metabolism is the slowest way to resynthesize AT ...
ReviewExamIII
... called the Calvin Cycle)? What is the function of NAD+ and FAD+ in cellular respiration? Why do glycolysis and the Krebs Cycle stop running when oxygen is lacking? How does fermentation allow glycolysis to start up again even in the absence of oxygen? Where in aerobic cellular respiration is the mos ...
... called the Calvin Cycle)? What is the function of NAD+ and FAD+ in cellular respiration? Why do glycolysis and the Krebs Cycle stop running when oxygen is lacking? How does fermentation allow glycolysis to start up again even in the absence of oxygen? Where in aerobic cellular respiration is the mos ...
Document
... To log onto the website, use the access code provided in your textbook. You will also find other resources, such as downloadable MP3 tutorials for each chapter, a glossary, and an electronic copy of your text- you can catch up on your reading anywhere! KEY TERMS ...
... To log onto the website, use the access code provided in your textbook. You will also find other resources, such as downloadable MP3 tutorials for each chapter, a glossary, and an electronic copy of your text- you can catch up on your reading anywhere! KEY TERMS ...
Chapter 6-7 Review Game
... A. Glucose is cycled around and resynthesized B. NAD+ and FAD are recycled C. The two-carbon acetyl CoA binds to a four-carbon molecule that is restored at the end of the cycle to be used again in the Citric Acid Cycle D. Carbon dioxide is cycled back to photosynthesis E. NADH is cycled down the ele ...
... A. Glucose is cycled around and resynthesized B. NAD+ and FAD are recycled C. The two-carbon acetyl CoA binds to a four-carbon molecule that is restored at the end of the cycle to be used again in the Citric Acid Cycle D. Carbon dioxide is cycled back to photosynthesis E. NADH is cycled down the ele ...
Anaerobic Fermentation
... Your body doesn't always get enough oxygen during excercise... *Body compensates for the lack of oxygen by a process called Anaerobic fermentation that carries out a series of chemical reactions that produce ATP from glucose in the absence of O 2 *Fermentation allows glycolysis to continue maki ...
... Your body doesn't always get enough oxygen during excercise... *Body compensates for the lack of oxygen by a process called Anaerobic fermentation that carries out a series of chemical reactions that produce ATP from glucose in the absence of O 2 *Fermentation allows glycolysis to continue maki ...
Chapter 9 Cellular Respiration: Harvesting Chemical Energy
... • Location --inside mitochondria “One-Two Punch” – Carbonyl group released as CO2 – NAD+ reduced to NADH – Leaves Acetyl--picked up by CoA & becomes Acetyl CoA ...
... • Location --inside mitochondria “One-Two Punch” – Carbonyl group released as CO2 – NAD+ reduced to NADH – Leaves Acetyl--picked up by CoA & becomes Acetyl CoA ...
PLANT – MICROBE INTERACTIONS Plant
... between two species, a phenomenon called syntrophism. Many micro organic synthesize the vitamins and anaerobic acids in excess of their nutritional requirements. Others have a requirement of one or more of these nutrients. Hence certain combinations of species will grow together but not apart when n ...
... between two species, a phenomenon called syntrophism. Many micro organic synthesize the vitamins and anaerobic acids in excess of their nutritional requirements. Others have a requirement of one or more of these nutrients. Hence certain combinations of species will grow together but not apart when n ...
UNIT 2: ECOLOGICAL BIOCHEMISTRY 2C: CHEMISTRY OF
... 1. The sun is the ultimate source of energy for most ecosystems. As a result, organisms exhibit different strategies to obtain this energy (directly or indirectly). 2. Energy relationships can be represented in a graphical depiction called a pyramid. 3. There are 2 major types of biological molecule ...
... 1. The sun is the ultimate source of energy for most ecosystems. As a result, organisms exhibit different strategies to obtain this energy (directly or indirectly). 2. Energy relationships can be represented in a graphical depiction called a pyramid. 3. There are 2 major types of biological molecule ...
Energy Review - MrsAllisonMagee
... What is inhibition? Define, list, describe • Chemicals that prevent enzyme activity • Competitive: binds to the active site • Non-competitive: binds somewhere else and changes the shape ...
... What is inhibition? Define, list, describe • Chemicals that prevent enzyme activity • Competitive: binds to the active site • Non-competitive: binds somewhere else and changes the shape ...
Document
... The Carbon Cycle o The reciprocal processes of photosynthesis and respiration provide a link between the atmosphere and the terrestrial environments. o Carbon recycles relatively fast because plants have a high demand for CO2. o Decomposition eventually recycles carbon to the atmosphere. o The amoun ...
... The Carbon Cycle o The reciprocal processes of photosynthesis and respiration provide a link between the atmosphere and the terrestrial environments. o Carbon recycles relatively fast because plants have a high demand for CO2. o Decomposition eventually recycles carbon to the atmosphere. o The amoun ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)