* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Populations and their Ecosystems
Survey
Document related concepts
Transcript
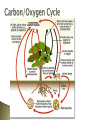
Populations and their Ecosystems Universe Galaxies Solar systems Biosphere Planets Earth Biosphere Ecosystems Ecosystems Communities Populations Organisms Organ systems Realm of ecology Communities Organs Tissues Cells Populations Protoplasm Molecules Atoms Subatomic Particles Organisms Fig. 3-2, p. 51 Human Affect On Condensation We move and store it Irrigation We drink it We treat water We pollute water ◦ ◦ ◦ ◦ Ground Water Uses: life, irrigation, swim/recreation, fish, animal habitat, weather Sewage Factories Feedlots Farm/fertilizer run-off Biggest Effect: Surface contamination putting excess nitrates and Phosphates in waterways – cause Algae blooms – called eutrophication All living things are made of carbon. Plants use carbon dioxide and sunlight to make their own food and grow. The carbon becomes part of the plant. Plants that die and are buried may turn into fossil fuels made of carbon like coal and oil over millions of years When humans burn fossil fuels, most of the carbon quickly enters the atmosphere as carbon dioxide. Human Affect On Diagram Burning fossil fuels puts excess Carbon in the atmosphere Deforestation reduces photosynthesizing plants; clears them faster than they can be replaced http://www.youtube.com/wat ch?v=w03iO_Yu9Xw&feature= related Most abundant element in the atmosphere 78% N2 = unreactive Nitrogen Gas Crucial component of proteins, vitamins, and nucleic acids (eg. DNA) Can not be absorbed directly from complex organisms (plants cannot take it from the air) Nitrogen fixing bacteria is crucial to convert Nitrogen into usable nutrients for plants and animals How Humans Affect It Diagram ◦ We add NO to air when we burn fuel – combines & falls as acid rain ◦ Add N20, from fertilizers and cattle ◦ NO3 - nitrates are added to soil from inorganic fertilizers; contaminates ground water ◦ Deforestation releases stored Nitrogen ◦ Excess Nitrates runoff into aquatic habitats through agri and sewage ◦ Nitrogen is removed from topsoil when we harvest crops, or burn and clear grasslands The Nitrogen Cycle All life requires nitrogen-compounds, e.g., proteins and nucleic acids. Air, which is 79% nitrogen gas (N2), is the major reservoir of nitrogen. But most organisms cannot use nitrogen in this form. Plants must secure their nitrogen in "fixed" form, i.e., incorporated in compounds such as: ◦ nitrate ions (NO3−) ◦ ammonia (NH3) ◦ urea (NH2)2CO Animals secure their nitrogen (and all other) compounds from plants (or animals that have fed on plants). We alter the nitrogen cycle by: Adding gases that contribute to acid rain. Adding nitrous oxide to the atmosphere through farming practices which can warm the atmosphere and deplete ozone. Contaminating ground water from nitrate ions in inorganic fertilizers. Releasing nitrogen into the troposphere through deforestation. Slow Moving in nature Increased by Human Action Key component of DNA Key component of Energy Storage Molecules Building Block Components of teeth & bones Slow Cycle that circulates in the soil and water Typically found as ocean sediments, in compounds such as salts that weather out of solid rock How Humans Affect Diagram We mine rock to make inorganic fertilizers & detergents We reduce phosphate available to ecosystems in deforestation Disrupt aquatic systems with phosphates from animal waste, fertilizers, and sewage treatment We remove large amounts of phosphate from the earth to make fertilizer. We reduce phosphorous in tropical soils by clearing forests. We add excess phosphates to aquatic systems from runoff of animal wastes and fertilizers. Sulfur is one of the components that make up proteins and vitamins. Proteins consist of amino acids that contain sulfur atoms. Sulfur is important for the functioning of proteins and enzymes in plants, and in animals that depend upon plants for sulfur. Plants absorb sulfur when it is dissolved in water. Animals consume these plants, so that they take up enough sulfur to maintain their health. Most of the earth's sulfur is tied up in rocks and salts or buried deep in the ocean in oceanic sediments. Sulfur can also be found in the atmosphere. It enters the atmosphere through both natural and human sources. Natural recourses can be for instance volcanic eruptions, bacterial processes, evaporation from water, or decaying organisms. Uses and Human Impact Natural sulfur comes from volcanoes, geysers, and hot springs We add sulfur dioxide to the atmosphere by: Burning coal and oil Refining sulfur containing petroleum. Convert sulfur-containing metallic ores into free metals such as copper, lead, and zinc releasing sulfur dioxide into the environment.