Notes
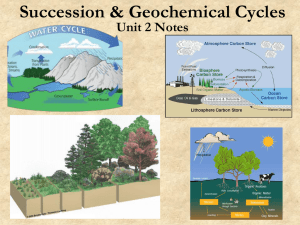
... The Nitrogen Cycle Nitrogen makes up 78% of the atmosphere but plants are unable to make use of this nitrogen gas and need a supply of ammonium or nitrate. The nitrogen cycle, a gaseous cycle, is dependent upon a number of bacteria. During nitrogen fixation, nitrogen-fixing bacteria living in nodul ...
... The Nitrogen Cycle Nitrogen makes up 78% of the atmosphere but plants are unable to make use of this nitrogen gas and need a supply of ammonium or nitrate. The nitrogen cycle, a gaseous cycle, is dependent upon a number of bacteria. During nitrogen fixation, nitrogen-fixing bacteria living in nodul ...
CH 3 Notes
... molecules that store genetic information in the cell 1. DNA (deoxyribonucleic acid): contains all the information for almost all cell activities 2. RNA (ribonucleic acid): stores and transfers information needed for making proteins 3. Nucleotides- linked monomers made up of ...
... molecules that store genetic information in the cell 1. DNA (deoxyribonucleic acid): contains all the information for almost all cell activities 2. RNA (ribonucleic acid): stores and transfers information needed for making proteins 3. Nucleotides- linked monomers made up of ...
Nutrition and Growth 2 Microbial Ecology
... is capable of growth in the absence of it Microaerophile: does not grow at normal atmospheric concentrations of oxygen but requires a small amount of it in metabolism Anaerobe: lacks the metabolic enzyme systems for using oxygen in respiration Strict, or obligate, anaerobes: also lack the enzymes fo ...
... is capable of growth in the absence of it Microaerophile: does not grow at normal atmospheric concentrations of oxygen but requires a small amount of it in metabolism Anaerobe: lacks the metabolic enzyme systems for using oxygen in respiration Strict, or obligate, anaerobes: also lack the enzymes fo ...
Biological Oceanography
... Habitat- the area where an organism lives Community- assortment of populations that live together in a specific place Population- a group of organisms of the same species living in the same ...
... Habitat- the area where an organism lives Community- assortment of populations that live together in a specific place Population- a group of organisms of the same species living in the same ...
Document
... a. CO2 and ATP are released during the process b. A multienzyme complex removes a carboxyl group, transfers electrons to NAD+, and attaches a coenzyme. c. NAD+ is rejuvenated so glycolysis can continue d. Lactate is produced to rejuvenate free NAD+ ...
... a. CO2 and ATP are released during the process b. A multienzyme complex removes a carboxyl group, transfers electrons to NAD+, and attaches a coenzyme. c. NAD+ is rejuvenated so glycolysis can continue d. Lactate is produced to rejuvenate free NAD+ ...
Problem Set# 3
... d. Anaerobes produces an extra FADH2 during the TCA cycle ______________________________________________________________________________ ______________________________________________________________ ...
... d. Anaerobes produces an extra FADH2 during the TCA cycle ______________________________________________________________________________ ______________________________________________________________ ...
Unit 13 - Electrochemistry
... the relationship between electric forces and chemical reactions. Voltage: The potential difference or electromotive force, measured in volts; it represents the amount of work that moving an electric charge between two points would take. Electrode: A conductor used to establish electrical contact wit ...
... the relationship between electric forces and chemical reactions. Voltage: The potential difference or electromotive force, measured in volts; it represents the amount of work that moving an electric charge between two points would take. Electrode: A conductor used to establish electrical contact wit ...
Document
... Location: Inner Membranes of Mitochondria Main Goal: Use hydrogen ions and electrons to make up to 34 ATP Process: -All NADH and FADH2 are electron carrier molecules - Made from glycolysis and krebs cycle NADH and FADH2 donate electrons and hydrogen ions to make ATP ...
... Location: Inner Membranes of Mitochondria Main Goal: Use hydrogen ions and electrons to make up to 34 ATP Process: -All NADH and FADH2 are electron carrier molecules - Made from glycolysis and krebs cycle NADH and FADH2 donate electrons and hydrogen ions to make ATP ...
Cellular Respiration
... Cellular respiration is the oxidative, chemical attack on energy-rich molecules to provide useful energy for the cell. Enzymes catalyze the oxidation reactions. These reactions are known as catabolic reactions because they break molecules down to release energy. Anaerobic respiration The first part ...
... Cellular respiration is the oxidative, chemical attack on energy-rich molecules to provide useful energy for the cell. Enzymes catalyze the oxidation reactions. These reactions are known as catabolic reactions because they break molecules down to release energy. Anaerobic respiration The first part ...
biology - OoCities
... is formed, oxidation occurs, nitrate is formed, more oxidation occurs, more nitrate is formed, then it can follow this path through another plant again or go to bacteria. This nitrogen cycle is not the only one present in an ecosystem that returns complex molecules to simple and simple to complex ov ...
... is formed, oxidation occurs, nitrate is formed, more oxidation occurs, more nitrate is formed, then it can follow this path through another plant again or go to bacteria. This nitrogen cycle is not the only one present in an ecosystem that returns complex molecules to simple and simple to complex ov ...
5.2 Describe species as reproductively distinct groups of organisms
... A Food Chain models the flow of energy through organisms in a community along a linear pathway. Organisms within a food chain are assigned to different levels within a food chain known as Trophic Levels. Trophic Levels consist of groups of organisms that have the same source of energy (a step in a ...
... A Food Chain models the flow of energy through organisms in a community along a linear pathway. Organisms within a food chain are assigned to different levels within a food chain known as Trophic Levels. Trophic Levels consist of groups of organisms that have the same source of energy (a step in a ...
File
... ecosystems, are capable of converting nitrogen found in the air or dissolved in water into the forms that are available for use by plants. • Intake of nitrogen into the organisms: Plants take in the nitrogen through their root systems in the form of ammonia or nitrate and in this way, nitrogen can e ...
... ecosystems, are capable of converting nitrogen found in the air or dissolved in water into the forms that are available for use by plants. • Intake of nitrogen into the organisms: Plants take in the nitrogen through their root systems in the form of ammonia or nitrate and in this way, nitrogen can e ...
Cellular Respiration
... CK ?? Topic CR Date: 10-11-10 4. How many usable ATP result from Glycolysis? 5. What is the second stage of CR? 6. Where does the second stage take place? 7. What does the term aerobic mean? ...
... CK ?? Topic CR Date: 10-11-10 4. How many usable ATP result from Glycolysis? 5. What is the second stage of CR? 6. Where does the second stage take place? 7. What does the term aerobic mean? ...
Cellular Respiration Guided Reading Notes Section 7
... 18. Table _____________________________ and ____________________ are made by yeast during alcoholic fermentation. 19. One molecule of sugar produces _________________ kilocalories of energy. 20. ________________________ respiration, like glycolysis, produces less energy than ________________________ ...
... 18. Table _____________________________ and ____________________ are made by yeast during alcoholic fermentation. 19. One molecule of sugar produces _________________ kilocalories of energy. 20. ________________________ respiration, like glycolysis, produces less energy than ________________________ ...
Citric Acid Cycle 2
... the first round of the citric acid cycle that could possibly release a carbon atom originating from this acetyl CoA? A) First round. B) Second round. C) Third round. D) Fourth round. 3. What type of enzyme is involved in all four redox reactions of the citric acid cycle? ...
... the first round of the citric acid cycle that could possibly release a carbon atom originating from this acetyl CoA? A) First round. B) Second round. C) Third round. D) Fourth round. 3. What type of enzyme is involved in all four redox reactions of the citric acid cycle? ...
Cellular Respiration
... 10. Fermentation is not as energy productive as respiration because a. it does not take place in a specialized membrane-bound organelle. b. it takes place within the mitochondria of cells. c. it is the pathway common to fermentation and respiration. d. NAD+ is regenerated by alcohol or lactate produ ...
... 10. Fermentation is not as energy productive as respiration because a. it does not take place in a specialized membrane-bound organelle. b. it takes place within the mitochondria of cells. c. it is the pathway common to fermentation and respiration. d. NAD+ is regenerated by alcohol or lactate produ ...
NME2.31 - Energy Production
... NADH is oxidised to NAD donating 2 electrons to the transport chain NADH ↔ NAD+ + H+ + 2e 4 protons are pumped into the inter-membrane space FADH2 dehydrogenase FADH2 is oxidised to FAD donating 2 electrons to the transport chain FADH2 ↔ FAD + 2H+ + 2e Ubiquinone transports electrons betwe ...
... NADH is oxidised to NAD donating 2 electrons to the transport chain NADH ↔ NAD+ + H+ + 2e 4 protons are pumped into the inter-membrane space FADH2 dehydrogenase FADH2 is oxidised to FAD donating 2 electrons to the transport chain FADH2 ↔ FAD + 2H+ + 2e Ubiquinone transports electrons betwe ...
Mark scheme Outline the process of glycolysis. (5 marks) occurs in
... inner membrane contains ATP synthetase / ATPase / stalked particles that make ATP (narrow) gap between inner and outer membranes / inter-membrane space ( must be stated or labeled) pH / H+ / proton concentration gradient rapidly established / steeper chemiosmosis therefore more efficient / chemiosmo ...
... inner membrane contains ATP synthetase / ATPase / stalked particles that make ATP (narrow) gap between inner and outer membranes / inter-membrane space ( must be stated or labeled) pH / H+ / proton concentration gradient rapidly established / steeper chemiosmosis therefore more efficient / chemiosmo ...
Food Webs - Highline Public Schools
... of a particular group of organisms. Depends on both size and population Energy – the amount of energy that can be obtained by eating a particular organism. The lowest level of an ecosystem always contains the most biomass and chemical energy. The highest level always has the least of both. ...
... of a particular group of organisms. Depends on both size and population Energy – the amount of energy that can be obtained by eating a particular organism. The lowest level of an ecosystem always contains the most biomass and chemical energy. The highest level always has the least of both. ...
File
... 9) Explain why 2 pyruvates are formed from one glucose molecule instead of only one pyruvate. 10) Name the organelle where aerobic respiration occurs? 11) During aerobic respiration, carbon dioxide (CO2) i ...
... 9) Explain why 2 pyruvates are formed from one glucose molecule instead of only one pyruvate. 10) Name the organelle where aerobic respiration occurs? 11) During aerobic respiration, carbon dioxide (CO2) i ...
Ecosystems: What Are They and How Do They Work
... 1. Autotrophs make their own food from compounds in the environment. 2. Consumers, or heterotrophs, feed on other organisms or their remains. a. Decomposers break down organic detritus (bacteria/fungi) into simpler inorganic compounds. b. Omnivores feed on both plants and animals. c. Carnivores feed ...
... 1. Autotrophs make their own food from compounds in the environment. 2. Consumers, or heterotrophs, feed on other organisms or their remains. a. Decomposers break down organic detritus (bacteria/fungi) into simpler inorganic compounds. b. Omnivores feed on both plants and animals. c. Carnivores feed ...
Cellular Respiration
... a. a series of chemical reactions that break down pyruvate, producing ATP , NADH & FADH2 that enter an electron transport chain b. the process in which energy from electrons in NADH and FADH2 is used to produce ATP; and water is produced. c. the process that breaks down glucose to pyruvate, producin ...
... a. a series of chemical reactions that break down pyruvate, producing ATP , NADH & FADH2 that enter an electron transport chain b. the process in which energy from electrons in NADH and FADH2 is used to produce ATP; and water is produced. c. the process that breaks down glucose to pyruvate, producin ...
Answer Key 2016 Spring Biology (General) Exam #2
... A) split a water molecule B) energize an electron C) produce ATP D) synthesize glucose 16) Plants produce oxygen when they photosynthesize. Where does the oxygen come from? A) splitting water molecules B) ATP synthesis C) the electron transport chain D) chlorophyll 17) Which color(s) of light does c ...
... A) split a water molecule B) energize an electron C) produce ATP D) synthesize glucose 16) Plants produce oxygen when they photosynthesize. Where does the oxygen come from? A) splitting water molecules B) ATP synthesis C) the electron transport chain D) chlorophyll 17) Which color(s) of light does c ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)