atomic mass
... Uses of Transition Elements • Most transition metals have higher melting points. • The filaments of light bulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3,410°C) and will not melt when a current ...
... Uses of Transition Elements • Most transition metals have higher melting points. • The filaments of light bulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3,410°C) and will not melt when a current ...
Atomic Structure - Tumwater School District
... outside – Zooming near the speed of light, with very little mass – Require more space than the nucelus ...
... outside – Zooming near the speed of light, with very little mass – Require more space than the nucelus ...
Physical Science
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
... mixtures of isotopes. Isotopes are atoms of the same element that differ in the number of neutrons. ...
Atoms, Electrons and Periodicity test - A
... Explain why the first ionisation energy of B is less than that of Be. ...
... Explain why the first ionisation energy of B is less than that of Be. ...
GHW - Louisiana Tech University
... Of course if we used some other mass unit for the mole such as "pound mole", the "number" would be different than 6.022 x 1023. 21) Given 5 moles of Sulfuric Acid having a formula of H2SO4 answer the following questions: ...
... Of course if we used some other mass unit for the mole such as "pound mole", the "number" would be different than 6.022 x 1023. 21) Given 5 moles of Sulfuric Acid having a formula of H2SO4 answer the following questions: ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... • The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is ...
... • The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The atomic mass is ...
atom - West Ada
... element’s name. The first letter is always capitalized and any other letter is not. Aluminum (Al), Platinum (Pt) and Cadmium (Cd) are some examples. The origins of some elements are not as obvious as others. Gold (Au) refers to the Latin name for gold, aurum. Lead (Pb) comes from the Latin word plub ...
... element’s name. The first letter is always capitalized and any other letter is not. Aluminum (Al), Platinum (Pt) and Cadmium (Cd) are some examples. The origins of some elements are not as obvious as others. Gold (Au) refers to the Latin name for gold, aurum. Lead (Pb) comes from the Latin word plub ...
Name Date Class DEFINING THE ATOM Section Review Objectives
... 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. 12. The atomic number of an atom is the total number of protons in an atom of that element. 13. An atom of nitrogen has 7 protons and 7 neutrons. 14. Relative atomic masses are expressed in amus. ...
... 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. 12. The atomic number of an atom is the total number of protons in an atom of that element. 13. An atom of nitrogen has 7 protons and 7 neutrons. 14. Relative atomic masses are expressed in amus. ...
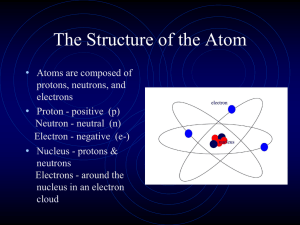
Chapter 4 Atomic Structure
... • Dalton’s atomic theory states that all atoms of a given element are identical. This is mostly true • Atoms of the same element can differ in the number of neutrons • most elements have two or more isotopes • Isotopes are atoms of the same element with different numbers of neutrons (and therefore d ...
... • Dalton’s atomic theory states that all atoms of a given element are identical. This is mostly true • Atoms of the same element can differ in the number of neutrons • most elements have two or more isotopes • Isotopes are atoms of the same element with different numbers of neutrons (and therefore d ...
Review
... 1. All hydrogen atoms contain one _____________________. However, like many naturally occurring elements, hydrogen can contain different numbers of _______________________. _________ types of hydrogen atoms are known. a. The most common type is sometimes called _______________________. Its nucleus h ...
... 1. All hydrogen atoms contain one _____________________. However, like many naturally occurring elements, hydrogen can contain different numbers of _______________________. _________ types of hydrogen atoms are known. a. The most common type is sometimes called _______________________. Its nucleus h ...
Isotopes - Ms. Bergman`s Classes at DCIS Montbello
... Calculating the average atomic mass Isotope Copper-63 Copper-65 ...
... Calculating the average atomic mass Isotope Copper-63 Copper-65 ...
Year 9 Science revison _15-16_ end of year CHEM
... i) How many neutrons does the Rb-87 isotope have ? 87 is the atomic mass. The atomic number is 37 for all isotopes of rubidium. ...
... i) How many neutrons does the Rb-87 isotope have ? 87 is the atomic mass. The atomic number is 37 for all isotopes of rubidium. ...
elements in a family have the same number of
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
Unit 3-The Atom Chapter Packet
... _____________________________3. proposed the first atomic model that accounted for the electrical nature of the atom _____________________________4. measured the size of the charge on an electron _____________________________5. suggested that alpha particles might be rebounding at an angle approachi ...
... _____________________________3. proposed the first atomic model that accounted for the electrical nature of the atom _____________________________4. measured the size of the charge on an electron _____________________________5. suggested that alpha particles might be rebounding at an angle approachi ...
Chapter 4 Atomic Structure
... Based on his experimental evidence: The atom is mostly empty space All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed aro ...
... Based on his experimental evidence: The atom is mostly empty space All the positive charge, and almost all the mass is concentrated in a small area in the center. He called this a “nucleus” The nucleus is composed of protons and neutrons (they make the nucleus!) The electrons distributed aro ...
Study Guide Answer Key
... 2. Consider an element Z that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 19.0 is 55.0% abundant; the isotope with a mass number of 21.0 is 45.0% abundant. What is the average atomic mass for element Z? [(mass A) (%A)] + [(mass B) (%B ...
... 2. Consider an element Z that has two naturally occurring isotopes with the following percent abundances: the isotope with a mass number of 19.0 is 55.0% abundant; the isotope with a mass number of 21.0 is 45.0% abundant. What is the average atomic mass for element Z? [(mass A) (%A)] + [(mass B) (%B ...
Distinguishing Between Atoms
... any one element are different from those of any other element. • Atoms of different elements can physically mix together or chemically combine with one another in simple whole number ratios to form compounds.(law of definite composition) • Chemical rxns occur when atoms are separated, joined, or rea ...
... any one element are different from those of any other element. • Atoms of different elements can physically mix together or chemically combine with one another in simple whole number ratios to form compounds.(law of definite composition) • Chemical rxns occur when atoms are separated, joined, or rea ...
Unit 2- The Atom
... always contains exactly the same proportions of the elements by weight. This law started being called Proust’s Law and is now named the Law of definite Proportion. John Dalton (1766‐1844) found the Law of Multiple Proportions that described compounds. This law stated that two elements form a s ...
... always contains exactly the same proportions of the elements by weight. This law started being called Proust’s Law and is now named the Law of definite Proportion. John Dalton (1766‐1844) found the Law of Multiple Proportions that described compounds. This law stated that two elements form a s ...
IT IS ELEMENTARY - the OLLI at UCI Blog
... • Pre 20th century almost all military poisons were of plant or animal origin—Sulfur was the exception • 1914-1918 A “golden age” for the military use of chemicals—the first weapons of mass destruction • Since the 1930s Chemical weapons have been used against defenseless peoples in colonial or civil ...
... • Pre 20th century almost all military poisons were of plant or animal origin—Sulfur was the exception • 1914-1918 A “golden age” for the military use of chemicals—the first weapons of mass destruction • Since the 1930s Chemical weapons have been used against defenseless peoples in colonial or civil ...
Atomic Structure Powerpoint
... therefore 1 proton or 1 neutron = ~1 amu 1 amu = 1.6606 x 10 -24 grams Since the mass mostly depends on # protons and # neutrons, you’d think atomic mass would be a whole number, but it isn’t. How come? ...
... therefore 1 proton or 1 neutron = ~1 amu 1 amu = 1.6606 x 10 -24 grams Since the mass mostly depends on # protons and # neutrons, you’d think atomic mass would be a whole number, but it isn’t. How come? ...
Chem-130 Test Lecture
... Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomic number. Isotopes are atoms of the same ...
... Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomic number. Isotopes are atoms of the same ...
In 1869, Russia`s Dmitri Mendeleev and Germany`s Lothar Meyer
... were identical, Mendeleev is given the credit because he predicted the existence of undiscovered elements and left spaces for them. Mendeleev ...
... were identical, Mendeleev is given the credit because he predicted the existence of undiscovered elements and left spaces for them. Mendeleev ...
Einsteinium

Einsteinium is a synthetic element with symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein. Its most common isotope einsteinium-253 (half life 20.47 days) is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but at much smaller amounts, by bombarding heavy actinide elements with light ions. Owing to the small amounts of produced einsteinium and the short half-life of its most easily produced isotope, there are currently almost no practical applications for it outside of basic scientific research. In particular, einsteinium was used to synthesize, for the first time, 17 atoms of the new element mendelevium in 1955.Einsteinium is a soft, silvery, paramagnetic metal. Its chemistry is typical of the late actinides, with a preponderance of the +3 oxidation state; the +2 oxidation state is also accessible, especially in solids. The high radioactivity of einsteinium-253 produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watts per gram. Difficulty in studying its properties is due to einsteinium-253's conversion to berkelium and then californium at a rate of about 3% per day. The isotope of einsteinium with the longest half life, einsteinium-252 (half life 471.7 days) would be more suitable for investigation of physical properties, but it has proven far more difficult to produce and is available only in minute quantities, and not in bulk. Einsteinium is the element with the highest atomic number which has been observed in macroscopic quantities in its pure form, and this was the common short-lived isotope einsteinium-253.Like all synthetic transuranic elements, isotopes of einsteinium are very radioactive and are considered highly dangerous to health on ingestion.