Energy Flow in Marine Ecosystem
... These components are linked together in a closed cycle (Fig. 1) ...
... These components are linked together in a closed cycle (Fig. 1) ...
Thermodynamics - Christian Hill
... Thermodynamics is one of the most powerful techniques that scientists have developed to understand the natural world. The laws of thermodynamics provide a general and comprehensive framework within which to study the properties of macroscopic systems (composed of many atoms or molecules), such as te ...
... Thermodynamics is one of the most powerful techniques that scientists have developed to understand the natural world. The laws of thermodynamics provide a general and comprehensive framework within which to study the properties of macroscopic systems (composed of many atoms or molecules), such as te ...
7.2 Writing Chemical Equations
... Chemical reactions can be described in different ways: descriptions, word equations, skeleton equations, and balanced equations. In writing chemical equations, reactants are written to the left of the arrow and the products are written to the right. Separate reactants and products are separated by ...
... Chemical reactions can be described in different ways: descriptions, word equations, skeleton equations, and balanced equations. In writing chemical equations, reactants are written to the left of the arrow and the products are written to the right. Separate reactants and products are separated by ...
exo and endo experiments
... The Law of Conservation of Mass The Law of Conservation of Mass was officially established in the year 1789 by the French Chemist, Antoine Lavoisier. The Law of Conservation of Mass states that mass is neither lost nor gained in chemical reactions, it states that it simply changes form. For that rea ...
... The Law of Conservation of Mass The Law of Conservation of Mass was officially established in the year 1789 by the French Chemist, Antoine Lavoisier. The Law of Conservation of Mass states that mass is neither lost nor gained in chemical reactions, it states that it simply changes form. For that rea ...
Properties and Classification of Matter
... Physical Properties ◦ Describe the physical appearance and composition of the substance ◦ Boiling point, freezing point, malleability, ductility, color, state, solubility, crystal formation, conductivity and magnetism ...
... Physical Properties ◦ Describe the physical appearance and composition of the substance ◦ Boiling point, freezing point, malleability, ductility, color, state, solubility, crystal formation, conductivity and magnetism ...
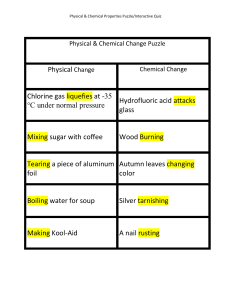
Physical Change Chlorine gas liquefies at
... Physical & Chemical Properties Puzzle/Interactive Quiz ...
... Physical & Chemical Properties Puzzle/Interactive Quiz ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
Science - St John`s School
... how and why decisions about science and technology are made the evidence for the origin, structure and continuing evolution of the Universe how the surface of the Earth and its atmosphere have changed since the Earth’s origin and are still changing the Earth’s crust, sea and atmosphere, and living o ...
... how and why decisions about science and technology are made the evidence for the origin, structure and continuing evolution of the Universe how the surface of the Earth and its atmosphere have changed since the Earth’s origin and are still changing the Earth’s crust, sea and atmosphere, and living o ...
chemical reaction?
... • What is an exothermic reaction? – A chemical reaction in which energy is released to the surroundings – Exothermic reactions often feel __________ because energy is released as heat – An example of an exothermic reaction is _______________ ...
... • What is an exothermic reaction? – A chemical reaction in which energy is released to the surroundings – Exothermic reactions often feel __________ because energy is released as heat – An example of an exothermic reaction is _______________ ...
Simple Chemical Reactions
... N4 Chemical change & structure - Energy changes of chemical reactions N4 Nature's Chemistry - Fuels N5 Nature's Chemistry - Energy from Fuels Revised Higher - Consumer Chemistry - 1c) Uses of alcohols ...
... N4 Chemical change & structure - Energy changes of chemical reactions N4 Nature's Chemistry - Fuels N5 Nature's Chemistry - Energy from Fuels Revised Higher - Consumer Chemistry - 1c) Uses of alcohols ...
Topic 2 The first law of thermodynamics
... State function is a property that is independent of how a sample is prepared, completely differential ,single valued Properties that relate to the preparation of the state are called path functions Question: T, P, V, ρ, Vm…… W, Q ...
... State function is a property that is independent of how a sample is prepared, completely differential ,single valued Properties that relate to the preparation of the state are called path functions Question: T, P, V, ρ, Vm…… W, Q ...
Thermodynamics
... Section 19.3 Molecules can undergo three kinds of motion: In translational motion the entire molecule moves in space. Molecules can also undergo vibrational motion, in which the atoms of the molecule move toward and away from one another in periodic fashion, and rotational motion, in which the entir ...
... Section 19.3 Molecules can undergo three kinds of motion: In translational motion the entire molecule moves in space. Molecules can also undergo vibrational motion, in which the atoms of the molecule move toward and away from one another in periodic fashion, and rotational motion, in which the entir ...
enthalpy worksheet
... Almost all chemical and physical reactions involve energy (usually in the form of heat) being released or added. An exothermic change is a reaction that releases energy. An endothermic change is one in which the energy must be added for the reaction to occur. For exothermic reactions, energy can be ...
... Almost all chemical and physical reactions involve energy (usually in the form of heat) being released or added. An exothermic change is a reaction that releases energy. An endothermic change is one in which the energy must be added for the reaction to occur. For exothermic reactions, energy can be ...
STUDY GUIDE for DIGESTION and NUTRITION
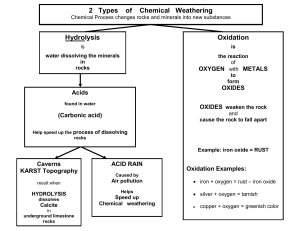
... Explain what indicators are used for and how they help determine if a substance is acidic or basic. Explain the significance of the pH scale. Find the names and formulas of common acids in your data booklet (e.g. HCl, H2SO4,) Chemical Reactions Describe the difference between a physical and ...
... Explain what indicators are used for and how they help determine if a substance is acidic or basic. Explain the significance of the pH scale. Find the names and formulas of common acids in your data booklet (e.g. HCl, H2SO4,) Chemical Reactions Describe the difference between a physical and ...
Chapter 15 - cloudfront.net
... • The pieces of rock that break off still have the same identity, they’re ...
... • The pieces of rock that break off still have the same identity, they’re ...
Matter - Wsfcs
... release of heat, light, odor, or sound. Examples: Burning (combustion), rusting, tarnishing and fermenting ...
... release of heat, light, odor, or sound. Examples: Burning (combustion), rusting, tarnishing and fermenting ...
Text Questions
... work except _____ work are done. 30. At constant pressure, the change in enthalpy equals… 31. For what two reasons is enthalpy a more useful function than internal energy? ...
... work except _____ work are done. 30. At constant pressure, the change in enthalpy equals… 31. For what two reasons is enthalpy a more useful function than internal energy? ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.