AP Chemistry Syllabus - HSANA AP Chemistry
... Assigned problems are begun in class and finished at home. Solutions to problems are reviewed in class in teacher-lead and student-lead sessions A spirit of family is created between students. Students are allowed at times to help or peer teach other students. All students in the class take the A ...
... Assigned problems are begun in class and finished at home. Solutions to problems are reviewed in class in teacher-lead and student-lead sessions A spirit of family is created between students. Students are allowed at times to help or peer teach other students. All students in the class take the A ...
this PDF file
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
PS5, Thermo Thermodynamics Standards: 3. Energy cannot be
... 9. Circle the letter of the source of the energy used by the heat engine to increase its internal energy. a. work output b. the sun c. low-temperature reservoir d. high-temperature reservoir 10. Is the following sentence true or false? Usable energy tends to become disorganized and unusable. 11. Cir ...
... 9. Circle the letter of the source of the energy used by the heat engine to increase its internal energy. a. work output b. the sun c. low-temperature reservoir d. high-temperature reservoir 10. Is the following sentence true or false? Usable energy tends to become disorganized and unusable. 11. Cir ...
Physical and Chemical Properties Worksheet Multiple Choice
... 13. Sodium reacts very easily with other ...
... 13. Sodium reacts very easily with other ...
Lecture 5
... • The number of degrees of freedom in a system is equal to the sum of the number of independent intensive variables (generally T and P) and independent concentrations of components in phases that must be fixed to define uniquely the state of the system. • A system that has no degrees of freedom is s ...
... • The number of degrees of freedom in a system is equal to the sum of the number of independent intensive variables (generally T and P) and independent concentrations of components in phases that must be fixed to define uniquely the state of the system. • A system that has no degrees of freedom is s ...
FHN - Chemical and Physical Changes
... change, but the substances in the material stay the same. Change in state Solid melting to a liquid Liquid evaporating to a gas Gas condensing to a liquid Liquid freezing into a solid Usually occur with a change in temperature Can also be when a substance dissolves in a liquid, but doe ...
... change, but the substances in the material stay the same. Change in state Solid melting to a liquid Liquid evaporating to a gas Gas condensing to a liquid Liquid freezing into a solid Usually occur with a change in temperature Can also be when a substance dissolves in a liquid, but doe ...
Chapter 19 Chemical Thermodynamics
... away from equil, sign of !G tells which way rxn goes Chemical ...
... away from equil, sign of !G tells which way rxn goes Chemical ...
Chapter 19 Chemical Thermodynamics
... This equation shows how G changes with temperature. (We assume S° & H° are independent of T.) Chemical Thermodynamics ...
... This equation shows how G changes with temperature. (We assume S° & H° are independent of T.) Chemical Thermodynamics ...
Chemical Reactions and The Mole Review
... • Focus question: What is the law of conservation of mass and what does it have to do with balancing chemical equations? • As you watch the video, jot down your thoughts on the focus question under your catalyst. Then, be ready to share. ...
... • Focus question: What is the law of conservation of mass and what does it have to do with balancing chemical equations? • As you watch the video, jot down your thoughts on the focus question under your catalyst. Then, be ready to share. ...
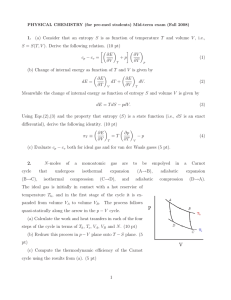
1. (a) Consider that an entropy S is as function of temperature T and
... from each other as well. Let the heat capacity of each gas be C1 and C2 . Box 1 starts with temperature Ti,1 , whereas box 2 starts with Ti,2 . (The subscript “i” means “initial,” and “f ” will mean “final.”) Assume that both gases are ideal so that the internal energy is given by Ei (T ) = Ci T . N ...
... from each other as well. Let the heat capacity of each gas be C1 and C2 . Box 1 starts with temperature Ti,1 , whereas box 2 starts with Ti,2 . (The subscript “i” means “initial,” and “f ” will mean “final.”) Assume that both gases are ideal so that the internal energy is given by Ei (T ) = Ci T . N ...
Chemical Synthesis
... • 1. Separate product from reaction mixture. • 2. Work out quantities of reactants needed. • 3. Purify the product. ...
... • 1. Separate product from reaction mixture. • 2. Work out quantities of reactants needed. • 3. Purify the product. ...
ppt
... • These are molar entropy values of substances in their standard states. • Standard entropies tend to increase with increasing molar mass. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
... • These are molar entropy values of substances in their standard states. • Standard entropies tend to increase with increasing molar mass. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
Final exam questions for Chemical Engineer BSc
... 11. Thermodynamics of multiphase and multicomponent system: Component, phases, degrees of freedoms. The phase rule (Gibbs-law) and its application to pure substances and two component systems. Liquid-liquid phase diagrams. Liquid-solid phase diagrams. Eutectics. 12. Chemical equilibrium: The reactio ...
... 11. Thermodynamics of multiphase and multicomponent system: Component, phases, degrees of freedoms. The phase rule (Gibbs-law) and its application to pure substances and two component systems. Liquid-liquid phase diagrams. Liquid-solid phase diagrams. Eutectics. 12. Chemical equilibrium: The reactio ...
A simple calorimeter was used as a vessel to measure the heat
... c) In the reaction above the initial temperature is 21.0˚C and the final temperature is 65.8˚C. Assume the density of KBr(aq) is 1.0g/mL. i. Calculate ΔT for this reaction ii. Clearly identify this reaction as exothermic or endothermic with the appropriate sign. ...
... c) In the reaction above the initial temperature is 21.0˚C and the final temperature is 65.8˚C. Assume the density of KBr(aq) is 1.0g/mL. i. Calculate ΔT for this reaction ii. Clearly identify this reaction as exothermic or endothermic with the appropriate sign. ...
Kémiai technológia I
... 11. Thermodynamics of multiphase and multicomponent system: Component, phases, degrees of freedoms. The phase rule (Gibbs-law) and its application to pure substances and two component systems. Liquid-liquid phase diagrams. Liquid-solid phase diagrams. Eutectics. 12. Chemical equilibrium: The reactio ...
... 11. Thermodynamics of multiphase and multicomponent system: Component, phases, degrees of freedoms. The phase rule (Gibbs-law) and its application to pure substances and two component systems. Liquid-liquid phase diagrams. Liquid-solid phase diagrams. Eutectics. 12. Chemical equilibrium: The reactio ...
SOL Essential Knowledge
... A. Recognize the following relative to the Kinetic Molecular Theory: 1. Atoms and molecules are in constant motion. 2. The theory is a model for predicting and explaining gas behavior. 3. Forces of attraction between molecules determine the physical changes of state. 4. Pressure, temperature and vol ...
... A. Recognize the following relative to the Kinetic Molecular Theory: 1. Atoms and molecules are in constant motion. 2. The theory is a model for predicting and explaining gas behavior. 3. Forces of attraction between molecules determine the physical changes of state. 4. Pressure, temperature and vol ...
PowerPoint for Cornell Notes
... The substance gaining electrons is said to be reduced and the substance losing the electrons is said to be oxidized. Thus an oxidation reaction is called a Redox reaction. In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its ...
... The substance gaining electrons is said to be reduced and the substance losing the electrons is said to be oxidized. Thus an oxidation reaction is called a Redox reaction. In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its ...
system
... Microscopic state of the system depends on the masses, velocities, positions, and all modes of motion of all of the constituent particles In the absence of detailed knowledge, thermodynamics considers the properties of the system which, when determined, define the macroscopic state of the system Sti ...
... Microscopic state of the system depends on the masses, velocities, positions, and all modes of motion of all of the constituent particles In the absence of detailed knowledge, thermodynamics considers the properties of the system which, when determined, define the macroscopic state of the system Sti ...
General Chemistry - Review for final exam: (Make sure you bring
... List the properties of the 3 states (phases) of matter. a. Solid b. Liquid c. Gas 5. What is the difference between a compound and a mixture? 6. What is a homogeneous mixture? 7. What is the name of a solid homogeneous mixture of metals? 8. What is a heterogeneous mixture? 9. What are the 2 pure sub ...
... List the properties of the 3 states (phases) of matter. a. Solid b. Liquid c. Gas 5. What is the difference between a compound and a mixture? 6. What is a homogeneous mixture? 7. What is the name of a solid homogeneous mixture of metals? 8. What is a heterogeneous mixture? 9. What are the 2 pure sub ...
$doc.title
... pharmaceutical industry involved particle technology, and formulation research and process development for oral dosage forms (tablets, capsules, suspensions) and transdermal formulations. She has developed and commercia ...
... pharmaceutical industry involved particle technology, and formulation research and process development for oral dosage forms (tablets, capsules, suspensions) and transdermal formulations. She has developed and commercia ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.