- Dr.Divan Fard
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. ...
... State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. ...
Chapter 7 - Chemical Reactions
... What volume is occupied by 0.75 moles of any gas at STP? How many moles of Cl2 are in 19.3 L of chlorine gas at STP? CHAPTER 10 OBJECTIVES Determine the specific heat of a material if a 10-gram sample absorbed 100 J as it was heated from 20ºC to 60ºC. How much heat is absorbed by a 200 gram piece of ...
... What volume is occupied by 0.75 moles of any gas at STP? How many moles of Cl2 are in 19.3 L of chlorine gas at STP? CHAPTER 10 OBJECTIVES Determine the specific heat of a material if a 10-gram sample absorbed 100 J as it was heated from 20ºC to 60ºC. How much heat is absorbed by a 200 gram piece of ...
File - Kheriaty Chemistry
... 22. a. Balance the reaction between sulfuric acid and ammonia (NH3) to yield ...
... 22. a. Balance the reaction between sulfuric acid and ammonia (NH3) to yield ...
C1a - Mr Corfe
... Q1 . Salts can be made by reacting acids with alkalis. This reaction is an example of? Q2. when potassium reacts with water the colour of the flame is? Q3. When baking powder is heated, it breaks down to form new substances. This is? Q4. A coin was reacted to form a solution. Sodium hydroxide soluti ...
... Q1 . Salts can be made by reacting acids with alkalis. This reaction is an example of? Q2. when potassium reacts with water the colour of the flame is? Q3. When baking powder is heated, it breaks down to form new substances. This is? Q4. A coin was reacted to form a solution. Sodium hydroxide soluti ...
15-7 Entropy and the Second Law of Thermodynamics
... (Equation 15.14: The Second Law of Thermodynamics) Entropy is sometimes referred to as time’s arrow – time proceeds in the direction of increasing entropy. Imagine watching a science fiction movie in which a spacecraft in deep space explodes into a million pieces. Then you play the film backwards, a ...
... (Equation 15.14: The Second Law of Thermodynamics) Entropy is sometimes referred to as time’s arrow – time proceeds in the direction of increasing entropy. Imagine watching a science fiction movie in which a spacecraft in deep space explodes into a million pieces. Then you play the film backwards, a ...
Energy and Chemical Reactions - Thermochemistry
... Chemical Potential Energy-The kind of Potential Energy of interest in Thermochemistry. Chemical potential energy results from attractive and repulsive forces between nuclei and electrons in a system. Includes the bond energies between atoms bonded together within a molecule (intramolecular), forces ...
... Chemical Potential Energy-The kind of Potential Energy of interest in Thermochemistry. Chemical potential energy results from attractive and repulsive forces between nuclei and electrons in a system. Includes the bond energies between atoms bonded together within a molecule (intramolecular), forces ...
Chapter 8
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
... • List three observations that suggest that a chemical reaction has taken place. • List three requirements for a correctly written chemical equation. • Write a word equation and a formula equation for a given chemical reaction. • Balance a formula equation by inspection. ...
Name__________________________ Period_______ Word
... (→) – this arrow indicated that the reactants are heated (s) – solid… all precipitates, powders, crystals or ashes are solids (l) – liquid…water is in the liquid state unless otherwise indicated) (g) – gas… all diatomic molecules are gases (H2, N2, F2, Cl2, Br2, Cl2, I2) (aq) – in aqueous solution ( ...
... (→) – this arrow indicated that the reactants are heated (s) – solid… all precipitates, powders, crystals or ashes are solids (l) – liquid…water is in the liquid state unless otherwise indicated) (g) – gas… all diatomic molecules are gases (H2, N2, F2, Cl2, Br2, Cl2, I2) (aq) – in aqueous solution ( ...
Midterm Review - Closter Public Schools
... _________________ form positive ions when they _______________ electrons. _________________form negative ions when they ________________ electrons. An ion with a +2 charge has ______________ 2 electrons. A _____________________ is the force of attraction between atoms that are sharing a pair of elec ...
... _________________ form positive ions when they _______________ electrons. _________________form negative ions when they ________________ electrons. An ion with a +2 charge has ______________ 2 electrons. A _____________________ is the force of attraction between atoms that are sharing a pair of elec ...
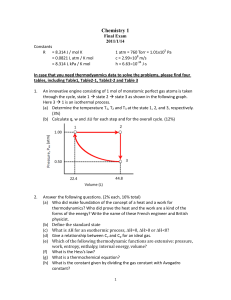
Chemistry 1
... The criterion for spontaneous process is G = H - TS < 0 at constant T and P. (a) For most combustion reactions, H < 0, and H overwhelms the contribution of the –TS term for the G being always negative. (b) For endothermic reactions, H > 0, the only way for G < 0 is TS > H, so S must be p ...
... The criterion for spontaneous process is G = H - TS < 0 at constant T and P. (a) For most combustion reactions, H < 0, and H overwhelms the contribution of the –TS term for the G being always negative. (b) For endothermic reactions, H > 0, the only way for G < 0 is TS > H, so S must be p ...
PHYSICAL CHEMISTRY ERT 108 Semester II 2010
... Aims- find the equilibrium composition of an ideal-gas reaction mixture and - Equilibrium composition is related to the initial composition by a single variable, the equilibrium extent of reaction ...
... Aims- find the equilibrium composition of an ideal-gas reaction mixture and - Equilibrium composition is related to the initial composition by a single variable, the equilibrium extent of reaction ...
chemistry 102 fall 2001 part 1
... (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decide between two answers, put one answer down for one question and the other answer down for the other question. If y ...
... (2) Each multiple choice question is actually 2 questions on your scanning sheet. If you are sure of an answer, put the same answer down for both questions for 5 pts. If you cannot decide between two answers, put one answer down for one question and the other answer down for the other question. If y ...
Atomic Weights Average Atomic Masses
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
... • Relative atomic mass: average masses of isotopes: – Naturally occurring C: 98.892 % 12C + 1.108 % 13C. • Average mass of C: • (0.98892)(12 amu) + (0.01108)(13.00335) = 12.011 amu. • Atomic weight (AW) is also known as average atomic mass (atomic weight). • Atomic weights are listed on the periodic ...
How to balance chemical equations.
... How to balance chemical equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coeffici ...
... How to balance chemical equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coeffici ...
Solid - burgess
... 1. Ions are atoms which have gained or lost electrons. 2. examples include Na+, Cl-, Mg2+, O2Try this online quiz on the organization of matter II. Physical and Chemical Properties A. Physical properties 1. properties that are determined by observation, either looking or measuring 2. examples includ ...
... 1. Ions are atoms which have gained or lost electrons. 2. examples include Na+, Cl-, Mg2+, O2Try this online quiz on the organization of matter II. Physical and Chemical Properties A. Physical properties 1. properties that are determined by observation, either looking or measuring 2. examples includ ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.