* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Chemical equilibrium wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Stoichiometry wikipedia , lookup
Thermodynamics wikipedia , lookup
Marcus theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Water splitting wikipedia , lookup
George S. Hammond wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Transition state theory wikipedia , lookup
Chapter Six:
THERMOCHEMISTRY
1
p228
Contents
2
p229
6-1 Nature of Energy
The Law of Conservation of Energy
Kinetic Energy
Pathway
Heat
State Function
Chemical Energy
Work
or State Property
Initial Position
In the initial position, ball A has a higher
potential energy than ball B.
Figure 6.1
4
After A has rolled down the hill, the potential
p230
energy lost by A has been converted to
random heading) and to the increase motions
of the components of the hill (frictional in the
potential energy of B.
Final Position
5
Final Position
After A has rolled down the hill, the
potential energy lost by A has been
converted to random motions of the
components of the hill (frictional heading)
and to the increase in the potential energy
of B.
6
System
p231
Surrounding
Exothermic: exo- is meaning “
out off”
; energy flows out of
the system.
Endothermic: the heat flow is into a system is endothermic.
Reactions that absorb energy from
surroundings are said to be endothermic.
7
Chemical Energy
p231
Figure 6.2
The combustion of methane releases the quantity of
energy Δ (PE) to surrounding via heat flow, This an
exothermic process.
8
The study of energy and its inter
conversions is called thermodynamics.
The conservation of energy is often called the first
law of thermodynamics. The energy of the universe
is constant.
p232
The internal energy of E of a system can be defined
most precisely as the sum of the kinetic and potential
energies of all the “
particles”in the system.
Surroundings
Surroundings
ΔE
=q+w
9
P233
Ex 6.1 Internal Energy
Calculate ΔE for a system undergoing an
endothermic process in which 15.6 kJ of heat flows
and where 1.4 kJ of work is done on the system.
Solution:
ΔE
=q+w
Where q = + 15.6 kJ, since the process is
endothermic, and w = + 1.4 kJ, since work is
done on the system. Thus
ΔE
= 1.56 kJ + 1.4 kJ = 17.0 kJ
10
p233
Work = force x distance = F x △h
Since P = F/A or F = P x A
Work = F x △h = P x A x △h
△V
= final volume –initial volume
= A x △h
Work = P x A x △h = P △V
W = - P △V
11
Ex 6.2 PV Work
P234
Calculate the work associated with the expansion of
a gas from 46 L to 64 L at a constant external
pressure of 15 atm.
Solution:
For a gas at constant pressure,
w = - PΔV
In this case P = 15 atm , and
ΔV = 64 - 46 = 18 L. Hence
w = -15 atm x 18 L = - 270 atm ∙L
12
Ex 6.3 Internal Energy, Heat, and Work
P234
A balloon is being inflated to its full extent by heating the
air inside it. In the final stages of this process, the volume
of the balloon changes from 4.00 × 106 L to 4.50 × 106 L by
the addition of 1.0 atm, calculate ΔE for the process. (To
convert between L˙atm = 101.3 J.)
Solution:
V = 4.50 x 106 L - 4.00 x 106 L = 0.50 x 106 L
Thus w = - (1.0 atm) x (5.0 x 105 L) x (101.3 J/(L· atm)
= - 5.1 x 107 J
Then E = q + w = (+1.3 x 108 J) + ( -5.1 x 107 J)
=8x
107
J
13
Ex 6.4 Enthalpy
P236
When 1 mole of methane (CH4) is burned at
constant pressure, 890 kJ of energy is released as
heat. Calculate ΔH for a process in which a 5.8-g
sample of methane is burned at constant pressure.
Solution:
5.8 g ÷(16 g/mole) = 0.36 mol CH4, and
0.36 mol x ( - 890 kJ/mol) = - 320 kJ
Thus, when 5.8-g Sample of CH4 is burned at
constant pressure,
ΔH = heat flow = -320 kJ
14
Coffee Creamer Flammability
15
Sugar and Potassium Chlorate
16
Thermite
17
1
t
c
a
Re
Is the freezing of water an endothermic or
exothermic process? Explain.
18
2
t
c
a
Re
Classify each process as exothermic or endothermic.
Explain.
a) Your hand gets cold when you touch ice.
b) The ice gets warmer when you touch it.
c) Water boils in a kettle being heated on a
stove.
d) Water vapor condenses on a cold pipe.
e) Ice cream melts.
19
Work vs. Energy Flow
20
6-2 Enthalpy and Calorimetry
Enthalpy
p235
H = E + PV
Change in H = (Change in E) + (Change in PV)
△E
= qp + W; △E = qp- P△V; qp = △E + P△V
△H
= △E + △ (PV); △(PV) = P △V
△H
= △E + P△V
qp =△E + P△V
△H
= qp
21
p236
ΔH = ΔE + Δ (PV), Δ(PV) = P ΔV
ΔH = ΔE + PΔV
qP = ΔE + PΔV
ΔH = qP
ΔH = Hproducts - Hreactants
22
Calorimetry
p237
Heat capacity
Specific heat capacity:
its unit L/K ‧ g
Molar heat capacity:
it has the units J/K‧ mol
Constant-pressure calorimetry
H+(aq) + OH-(aq) → H2O(l)
Figure 6-5
A Coffee Cup Calorimeter
Made of Two Styrofoam
Cups
23
Energy released by the neutralization reaction
p238
24
p242
Ex 6.6 Constant-Volume Calorimetry
p242
It has been suggested that hydrogen gas obtained
by the decomposition of water might be a substitute
for natural gas (principally methane). To compare
the energies of combustion of these fuels, the
following experiment was carried out using a bomb
calorimeter with a heat capacity of 11.3 kJ/℃.
When a 1.50-g sample of methane gas burned with
excess oxygen in the calorimeter, the temperature
increased by 7.3℃. When a 1.15-g sample of
hydrogen gas was burned with excess oxygen, the
temperature increase was 14.3℃. Calculate the
energy of combustion (per gram) for hydrogen and
26
oxygen.
Solution
Energy released in the combustion of 1.5 g CH4
= 11.3 kJ/℃)(7.3℃) = 8.3 kJ
Energy released in the combustion of 1 g CH4
= 83 kJ/(1.5 g) = 55 kJ/g
Similarly, for hydrogen
Energy released in the combustion of 1.15 g H2
= (11.3 kJ/℃)(14.3℃) = 162 kJ
Energy released in the combustion of 1 g H2
= 162 kJ/(1.15 g) = 141 kJ/g
27
3
t
c
a
Re
You have a Styrofoam cup with 50.0 g of water at
10
C. You add a 50.0 g iron ball at 90
C to the
water. The final temperature of the water is:
a) Between 50°C and 90°C.
b) 50°C
c) Between 10°C and 50°C.
Calculate the final temperature of the water.
28
p242
29
6-3 Hess’
s Law
p242
Figure 6-7 The principle of Hess’
slaw. The same change in
enthalpy occurs when nitrogen and oxygen react to form
nitrogen dioxide, regardless of whether the reaction occurs
in one (red) or two (blue) steps.
30
p243
N2(g) +2O2(g) → 2NO2 (g),
△H1 =
68 kJ
N2(g) + O2(g) → 2NO(g), ΔH2 = 180 kJ
2NO(g) + O2(g) → 2NO2(g), ΔH3 = -112 kJ
Net reaction: N2(g) + 2O2(g) → 2NO2(g), ΔH2 + ΔH3 = 68 kJ
ΔH1
= ΔH2 + ΔH3 = 68 kJ
31
Characteristic of Enthalpy Changes
p243
1. If a reaction is reversed, the sign of ΔH is also reversed.
2.The magnitude of ΔH is directly proportional to the
quantities of reactants and products in a reaction. If the
coefficients in a balanced reaction are multiplied by integer,
the value of ΔH is multiplied by the same integer.
Xe(g) + 2F2(g) → XeF4(s), ΔH = -251 kJ
XeF4(s) → Xe(g) + 2F2(g), ΔH = ?
Crystals of xenon tetrafluoride, the first reported
binary compound containing a noble gas element.
32
Hess’
s Law
33
Ex 6.7 Hess’
s Law
P244
Two forms of carbon are graphite, the soft, black,
slippery material used in “
lead”pencils and as a lubricant
for locks, and diamond, the brilliant, hard gemstone.
Using the enthalpies of combustion for graphite (-394
kJ/mol) and diamond (-396 kJ/mol), calculate ΔH for the
conversion of graphite to diamond: C graphite ( s) Cdiamond ( s )
Solution:
34
Hints for Using Hess’
s Law
p246
1. Work backward from the required reaction, using the
reactants and products to know to manipulate the
other given reactants at your disposal.
2. Reverse any reactions as needed to give the required
reactants and products.
3. Multiply reactions to give the correct numbers of
reactants and products.
35
p246
6-4 Standard Enthalpies of Formation
Cgraphite(s) → Cdiamond(s)
Standard enthalpy of formation is defined as the change
in enthalpy that accompanied the formation of one mole
of a compound from its elements with all substances in
their standard states.
A degree symbol on a thermodynamic function, for
example, ΔH0, indicates that the corresponding process
has been carried out under standard conditions.
36
Conventional Definitions of Standard states
p246
For a compound
1. The standard state of a gaseous substance is a pressure of
exactly 1 atmosphere.
2. For a pure substance in a condensed state (liquid or solid),
the standard state is the pure liquid or solid.
3. For a substance present in a solution, the standard is a
concentration of exactly 1 M.
For an Element
The standard state of an element is the which the element exists
under conditions of 1 atmosphere and 25 C.( The standard state for
oxygen is O2(g) at a pressure of 1 atmosphere; the standard state for
sodium is Ma(s); the standard for mercury is Hg(l); and so on. ) 37
CH 4 ( g ) 2O 2 ( g ) CO2 ( g ) H 2O(l ) H
o
reaction
C( s ) O 2 ( g ) CO2 ( g ) H fo 394 kJ/mol
CH 4 ( g ) C( s ) 2H 2 ( g )
C( s ) 2H 2 ( g ) CH 4 ( g ) ΔΗ0f 75 kJ/mol
1
H 2 ( g ) O 2 ( g ) H 2O(l ) H f0 286 kJ/mol
2
?
p247
A Schematic Diagram of the Energy Changes
for the Reaction
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
39
Change in enthalpy can be calculated from enthalpies
of formation of reactants and products.
p247
H°reaction = npHf
(products) - nrHf
(reactants) (6.1)
40
Standard States
Compound
For a gas, pressure is exactly 1 atmosphere.
For a solution, concentration is exactly 1 molar.
Pure substance (liquid or solid)
Element
The form [N2(g), K(s)] in which it exists at 1 atm
and 25°C.
41
P249
Ex 6.9 Enthalpies from Standard Enthalpies
of Formation
Using the standard enthalpies of formation listed in
Table 6.2, calculate the standard enthalpy change for
the overall reaction that occurs when ammonia is
burned in air to form nitrogen dioxide and water. This
is first step in the manufacture of nitric acid.
Solution:
4 NH 3 ( g ) 7O2 ( g ) 4 NO2 ( g ) 6 H 2O(l )
ΔH0reaction = { - 4 mol [-(46 kJ/mol)]} + [ - 7 mol ( 0
kJ/mol )] + [ 4 mol ( + 34 kJ/mol)] +
[ 6 mol ( - 286 kJ)]
= - 1396 kJ
42
Ex 6.11 Enthalpies from Standard of Formation III
p252
Methanol (CH3OH) is often used as fuel in high-performance
engines in race cars. Using the data in Table 6.2, compare the
standard enthalpy of combustion per gram of methanol with
that per gram of gasoline. Gasoline is actually a mixture of
compounds, but assume for this problem that gasoline is
liquid octane (C8H18).
Solution
2CH 3OH (l ) 3O 2 ( g ) 2CO 2 ( g ) 4H 2O(l )
43
For Methanol
For Octane
p252
6-5 Present Sources of Energy
Petroleum and
Natural Gas
p252
p257
Figure 6.14
p257
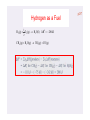
Hydrogen as a Fuel
1
H 2 (g) O2 (g) H 2O(l) ΔH0 286 kJ
2
CH 4 (g) H 2O(g) 3H 2 (g) CO(g)
CH 4 ( g ) H 2O ( g ) 3H
p259
Wind turbines to create electricity.
Ex 6.12 Enthalpies of Combustion
P260
Compare the energy available from the combustion of given
volume of methane and the same volume of hydrogen at the
same temperature and pressure.
Solution:
49
P261
Ex 6.13 Comparing Enthalpies of Combustion
Assuming that the combustion of hydrogen gas
provides three times as much energy per gram as
gasoline, calculate the volume of liquid H2 (density
= 0.0710 g/mL) required to furnish the energy
contained in 80.0 L (about 20 gal) of gasoline
(density = 0.740 g/mL). Calculate also the volume
that this hydrogen would occupy as a gas at 1.00
atm and 25℃.
50
Solution:
p261
Energy Sources Used in the United States
52
The Earth’
s
Atmosphere
53