AP Chemistry - Allen County Schools
... mathematically manipulating, graphing, evaluating, and reporting qualitative and quantitative data from their work. Lab scores include the pre-lab assignments, the skills exhibited in the laboratory, and the quality, accuracy, and completeness of the lab reports. Some experiments require the student ...
... mathematically manipulating, graphing, evaluating, and reporting qualitative and quantitative data from their work. Lab scores include the pre-lab assignments, the skills exhibited in the laboratory, and the quality, accuracy, and completeness of the lab reports. Some experiments require the student ...
Classification of Matter
... • A pure substance has a fixed composition and distinct properties • A mixture consists of two or more pure substances which retain their chemical identities. ...
... • A pure substance has a fixed composition and distinct properties • A mixture consists of two or more pure substances which retain their chemical identities. ...
Anonymous-IntroductiontoThermodynamics-qsp_chapte+
... 6.2 Reversible and Irreversible Processes We need to introduce an important, but subtle, idea here. We are interested in how a thermal system, such as an expansion, evolves from one state to another and hence how much work is done on or by the system and how much heat is exchanged with the surroundi ...
... 6.2 Reversible and Irreversible Processes We need to introduce an important, but subtle, idea here. We are interested in how a thermal system, such as an expansion, evolves from one state to another and hence how much work is done on or by the system and how much heat is exchanged with the surroundi ...
Classification of Matter
... • A pure substance has a fixed composition and distinct properties • A mixture consists of two or more pure substances which retain their chemical identities. ...
... • A pure substance has a fixed composition and distinct properties • A mixture consists of two or more pure substances which retain their chemical identities. ...
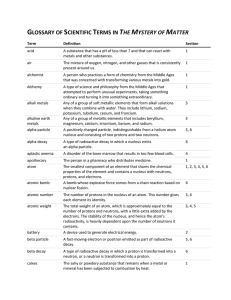
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER
... A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the six gases helium, neon, argon, krypton, xenon, and radon. Because the outermost el ...
... A group of two or more atoms linked together by sharing electrons in a chemical bond. A heavy, neutral particle in an atom’s nucleus that accounts for almost all of each atom’s mass, in addition to protons. Any of the six gases helium, neon, argon, krypton, xenon, and radon. Because the outermost el ...
AHSGE Review
... Groups are together because the elements in them have similar properties and react in the same manner. Across periods (left to right), atomic radius (size) decreases, ionization energy (ease of losing an electron) increases, and electronegativity (ability to attract electrons) increases. ...
... Groups are together because the elements in them have similar properties and react in the same manner. Across periods (left to right), atomic radius (size) decreases, ionization energy (ease of losing an electron) increases, and electronegativity (ability to attract electrons) increases. ...
Chapter 12 Stoichiometry - Conejo Valley Unified School
... • Stoichiometry is the part of chemistry that studies amounts of reactants and products that are involved in reactions. ...
... • Stoichiometry is the part of chemistry that studies amounts of reactants and products that are involved in reactions. ...
The Laws of Thermodinamics
... W= -P ΔV –can be used to calculate the work done on the system only when the pressure of the gas remain ct. during the expansion or compression ISOBARIC PROCESS- a process in which the pressure remain constant • The area under the graph in a PV diagram is equal in magnitude to do work done on a gas ...
... W= -P ΔV –can be used to calculate the work done on the system only when the pressure of the gas remain ct. during the expansion or compression ISOBARIC PROCESS- a process in which the pressure remain constant • The area under the graph in a PV diagram is equal in magnitude to do work done on a gas ...
Kinetics of the Selective Reaction of Diazonium Salts with Single
... Current methods of synthesis for carbon nanotubes (CNTs) usually produce heterogeneous mixtures of different nanotube diameters, thus a mixture of electronic properties. Consequently, many techniques have been developed in attempts to separate nanotubes according to their electronic type, w ith vary ...
... Current methods of synthesis for carbon nanotubes (CNTs) usually produce heterogeneous mixtures of different nanotube diameters, thus a mixture of electronic properties. Consequently, many techniques have been developed in attempts to separate nanotubes according to their electronic type, w ith vary ...
Name - Net Start Class
... 1. Define ‘extensive properties’ and give 3 examples a. Definition - a property that depends on how much matter is being considered. b. Ex. 1 Size c. Ex. 2 Mass d. Ex. 3 Volume 2. Define ‘intensive properties and give 3 examples. a. Definition - a property that does not depend on how much matter is ...
... 1. Define ‘extensive properties’ and give 3 examples a. Definition - a property that depends on how much matter is being considered. b. Ex. 1 Size c. Ex. 2 Mass d. Ex. 3 Volume 2. Define ‘intensive properties and give 3 examples. a. Definition - a property that does not depend on how much matter is ...
Thermochemistry 2 Matching Match each item with the correct
... Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
... Matching Match each item with the correct statement below. a. heat of reaction d. heat of fusion b. heat of formation e. heat of solution c. Hess's law of heat summation ____ ...
CH1101 2014/2015
... First Law of Thermodynamics: Internal energy U or E. Internal energy (U or E) : is the total energy of the system at any given time. Comes from the total kinetic and potential energy of molecules which compose the system. Change in internal energy (U or E) : energy change as system goes from an i ...
... First Law of Thermodynamics: Internal energy U or E. Internal energy (U or E) : is the total energy of the system at any given time. Comes from the total kinetic and potential energy of molecules which compose the system. Change in internal energy (U or E) : energy change as system goes from an i ...
Solutions to TI4: First Law of Thermodynamics
... 1. Consider the human body as a system and apply the first law of thermodynamics to it.. a. Internal energy is related to temperature. The human body has fairly constant temperature, hence the internal energy does not decrease as described above. b. Internal energy is added to the body to balance th ...
... 1. Consider the human body as a system and apply the first law of thermodynamics to it.. a. Internal energy is related to temperature. The human body has fairly constant temperature, hence the internal energy does not decrease as described above. b. Internal energy is added to the body to balance th ...
Notes
... Terms essential to the understanding of calorimetry are heat capacity and specific heat. Heat capacity is defined as the amount of heat necessary to raise the temperature of an object by one degree (Celsius or Kelvin). The units of heat capacity are joules per degree, J/°C or J/K. For pure substance ...
... Terms essential to the understanding of calorimetry are heat capacity and specific heat. Heat capacity is defined as the amount of heat necessary to raise the temperature of an object by one degree (Celsius or Kelvin). The units of heat capacity are joules per degree, J/°C or J/K. For pure substance ...
FXM Rev 1 Key - Grande Cache Community High School
... an element. This number is not found on the periodic table. organic chemistry This is the chemistry of carbon. It is the study of most carbon based compounds. endothermic reaction This is a chemical reaction that requires heat energy to be absorbed to take place. Photosynthesis is this type of react ...
... an element. This number is not found on the periodic table. organic chemistry This is the chemistry of carbon. It is the study of most carbon based compounds. endothermic reaction This is a chemical reaction that requires heat energy to be absorbed to take place. Photosynthesis is this type of react ...
Diapositivo 1
... But we have seen before that (recall the fundamental equations for open systems): ...
... But we have seen before that (recall the fundamental equations for open systems): ...
Thermodynamic functions - Phase Transformations Group
... It is evident that neither the enthalpy nor the entropy change can be used in isolation as reliable indicators of whether a reaction should occur ...
... It is evident that neither the enthalpy nor the entropy change can be used in isolation as reliable indicators of whether a reaction should occur ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.