* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Notes
Thermal radiation wikipedia , lookup
Calorimetry wikipedia , lookup
Heat exchanger wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
R-value (insulation) wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Heat capacity wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat equation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermal conduction wikipedia , lookup
Adiabatic process wikipedia , lookup
Heat transfer wikipedia , lookup
Thermodynamic system wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
5.1
The Nature of Energy
Energy can be defined as the capacity to do work or to transfer heat. Matter possesses
energy both by virtue of its motion (kinetic energy) and by virtue of its position (potential
energy). Thermochemistry is the study of the transfer of energy from one sample of
matter to another in the form of heat or work.
The SI unit of energy is the joule. One joule is the amount of kinetic energy possessed by
a 2 kilogram object moving at a speed of one meter per second:
The study of thermochemistry requires the definition of system and surroundings. The
system is the particular sample of matter under investigation, and the surroundings
include everything else outside the system.
5.2
The First Law of Thermodynamics
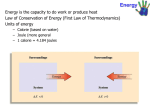
Although kinetic energy can be converted to potential energy, and vice versa, the total
amount of energy possessed by the system and surroundings remains constant. This is the
first law of thermodynamics
The change in internal energy associated with a process is related to heat and work by the
equation
where q is heat transferred and w is work done. By convention, when heat is transferred
to the system from the surroundings, q is a positive number. When heat flows from
system to surroundings, q is a negative number. Similarly, when the surroundings do
work on the system, w is positive, and when the system does work on the surroundings, w
is negative.
Most of the material in this chapter is concerned with heat transfer rather than work. An
exothermic process is one that results in heat being transferred from the system to the
surroundings. An endothermic process results in heat being transferred from the
surroundings to the system.
5.3
Enthalpy
The internal energy of a system can be divided into two parts: The capacity to do
pressure-volume work, – PV (recall that when work is done by the system, the sign of w
is negative); and the capacity to transfer heat, known as the enthalpy, H.
Like internal energy, enthalpy itself cannot be determined exactly, but changes in
enthalpy ( H) can be determined. Many chemical reactions are carried out at constant
pressure. For a constant-pressure process, the changes in internal energy and enthalpy are
related by the equation
In addition to being carried out at constant pressure, many reactions (particularly those
carried out in solution) exhibit a negligible volume change. In this case the term P V is
essentially zero, and the values of E and H are essentially equal. The change in
enthalpy, H, is the heat exchanged between system and surroundings as the result of a
process carried out at constant pressure: H = qP. According to the sign convention, an
exothermic process will have a negative H and an endothermic process will have a
positive H. Enthalpy, like internal energy, is a state function.
Enthalpy is an extensive property.
The enthalpy change of a reaction is equal in magnitude but opposite in sign to
the enthalpy change of the reverse reaction:
The enthalpy change of a reaction depends on the states of matter of the products
and reactants.
5.4
Enthalpies of Reaction
Also known as the heat of reaction, the enthalpy of reaction ( Hrxn) is the heat
exchanged between system and surroundings at constant pressure, as the result of a
chemical reaction. ( H's can also be written for physical processes, and a subscript
abbreviating the specific process is typically included. For example, the heat required to
vaporize a mole of substance is referred to as Hvap, the delta H of vaporization or the
heat of vaporization of that substance.)
If the reactants in a chemical reaction have a greater enthalpy (heat content) than the
products—the reactants have to give off heat in order to become products—the sign of
H will be negative. A negative H corresponds to an exothermic process. Conversely, a
positive H corresponds to an endothermic process.
Hsolution , sometimes called heat of solution or molar enthalpy of dissolution, refers to
the Hrxn for the physical process of dissolving one mole of a substance in water.
5.5
Calorimetry
Calorimetry is a method of measuring heat flow between systems and surroundings.
Terms essential to the understanding of calorimetry are heat capacity and specific heat.
Heat capacity is defined as the amount of heat necessary to raise the temperature of an
object by one degree (Celsius or Kelvin). The units of heat capacity are joules per degree,
J/°C or J/K. For pure substances the heat capacity is usually given in terms of a specified
amount of the substance. The heat capacity of 1 mol of a substance is called its molar
heat capacity. This is simply the amount of heat necessary to raise the temperature of 1
mol of a substance by one degree. The units of molar heat capacity are joules per mole
per degree, J/mol-°C or J/mol-K. Specific heat, also known as specific heat capacity, is
defined as the amount of heat necessary to raise the temperature of 1 g of a substance by
one degree. The units of specific heat are joules per gram per degree, J/g-°C or J/g-K.
Note that heat capacity applies to objects, while molar and specific heat capacities apply
to substances. Note also that the units of each can specify degrees Celsius or Kelvin,
since the magnitude of a degree is the same on both scales.
The device used to measure heat flow between systems and surroundings is called a
calorimeter. You will encounter two kinds of calorimetry: constant-pressure
calorimetry and constant-volume, or "bomb," calorimetry.
5.6
Hess's Law
Enthalpies of reaction values for a great many reactions have been determined and
tabulated. Some H values are difficult or impossible to determine directly. In many
cases Hess's law allows us to determine the H of a reaction without actually carrying
out the reaction.
Hess's law states that if we can add two or more equations to get the desired equation, we
can also add their H's to get the desired H. We can regard this reaction as one that
consists of two distinct steps, each of which has a particular H° associated with it.
5.7
Enthalpies of Formation
Using Hess's law allows us to determine H values for reactions without actually
performing them, as long as we have sufficient data about the steps that make up the
reactions. We do not always have all of the information necessary to do this. Fortunately,
there is another way to determine the heat of reaction without actually performing an
experiment.
H°f ("standard delta H of formation"; also called heat of formation) values are
tabulated for a large number of compounds. (Appendix C in your textbook contains H°f
values.) H°f is defined as the heat of reaction for the production of one mole of a
substance from its constituent elements, each in their standard states. For instance, the
H°f of liquid water is the H for the following reaction (at 298 K).
Up until this point you have been taught to balance equations using only whole numbers.
In this case, however, the coefficient for oxygen gas is a fraction. This is necessary in
order to balance the equation and have it produce only 1 mol of water. (Remember that
production of one mole is part of the definition of H°f.)
By definition, H f of any element in its standard state (the form that is most stable at
298 K and atmospheric pressure) is zero.
Using this information and the equation
where n and m represent the coefficients in a balanced chemical equation, we can
calculate the heat of a reaction without actually carrying out the reaction. (Often it is
desirable to know the magnitude of the H before attempting a reaction. A very large
positive H indicates that the reaction probably will be difficult to perform. A very large
negative H indicates that the reaction may be dangerous to perform.)
5.8
Foods and Fuels
The reactions involved in producing energy from food and those involved in the
combustion of fossil fuels are remarkably similar. Both ultimately convert carbon-based
compounds to carbon dioxide and water and produce energy in the process.
The digestion process breaks down the food we eat into "blood sugar" (glucose), which
combines with oxygen in what is essentially a controlled combustion process. (Without
the flame, of course!)
This process produces the heat necessary to maintain body temperature and function. Any
such energy produced in the body and not expended is stored as fat for future energy
production.
The energy unit Calorie is still commonly used to indicate the fuel content of foods.
Among proteins, carbohydrates, and fats (the principal constituents of a human diet), fats
provide the most energy per gram consumed. In other words, fats have a higher fuel
value than do either proteins or carbohydrates. Below is a table of various foods and their
fuel values.
Fossil fuels such as natural gas, petroleum, and coal can also be characterized by fuel
value. Here is a table of fuels—most of them petroleum—with their percentage
compositions and fuel values.