* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thermochemistry Energy - the capacity to do work Potential energy
Solar water heating wikipedia , lookup
Intercooler wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Thermoregulation wikipedia , lookup
Solar air conditioning wikipedia , lookup
Cogeneration wikipedia , lookup
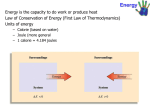
Thermochemistry Energy - the capacity to do work Potential energy - energy due to position or composition Kinetic energy - energy due to the motion of an object KE = 1/2 mν2 (m = mass ν = velocity) Joule - the SI unit of energy Thermochemistry • studies the energy changes that occur during chemical reactions. system - the part of the universe being studied surroundings - the rest of the universe open system - exchanges matter and energy with the surroundings closed system - exchanges energy but not matter with the surroundings isolated system - exchanges neither energy nor matter with the surroundings Internal Energy (U) - the total energy contained within the system Heat (q) - the energy transfer between the system and its surroundings Work (w) = force x distance (N x m = 1 kg m2s-2 = J) Pressure volume work w = -P∆V (pressure x change in volume) State Functions (functions of state) • • Properties that have a unique value and depend only on the present state of the system, not how that state was reached. Internal energy is a state function (q and w are not) Law of conservation of energy: In a physical or chemical change, energy can be exchanged between the system and its surroundings, but no energy can be created or destroyed. Thermodynamics - studies energy and its conversions First Law of Thermodynamics : ∆U = q + w Conventions: In chemistry, the signs for work, energy, heat etc, are usually from the system's point of view. Energy entering a system (endothermic) has a positive sign. Energy leaving a system (exothermic) has a negative sign. Heat absorbed by the system has a positive sign. Heat lost by the system has a negative sign. Work done on a system has a positive sign. Work done by the system has a negative sign. Note: these conventions are not the same for all fields; an engineer may define work from the surroundings point of view. Exothermic Reactions: • • • chemical energy in a system is converted to thermal energy in an isolated system the temperature of the system will increase. in an open or closed system, heat will be given off to the surroundings (q is negative). Note the temperature of the system may also increase initially. Endothermic Reactions: • • • thermal energy is converted to chemical energy in an isolated system the temperature of the system will decrease in an open or closed system, heat will be absorbed from the surroundings (q is positive) Heat of Reaction (qrxn) • • • the quantity of heat exchanged between the system and its surroundings for a reaction occurring at a fixed temperature. When a reaction is carried out at constant volume, DU = qv When a reaction is carried out at constant pressure, qp = DU + PDV Enthalpy (H): • • The sum of the internal energy and the pressure-volume product of the system. H = U + PV • • • • • • • • If a process is carried out at constant temperature and the only work done is pressurevolume work, then ∆H = qp = ∆U + P∆V Enthalpy is an extensive property - it depends on the quantities of substances present. Enthalpy is a function of state - it only depends on the present state, not on the pathway it took to get there. The difference in enthalpy between two states of a system has a unique value. For exothermic reactions ∆H is negative For endothermic reactions, ∆H is positive Enthalpy diagrams show enthalpy changes through a chemical reaction. Note: when a reaction is reversed, the enthalpy change has the same value but the opposite sign. Calorimetry: • • The measure of a quantity of heat Calorimeter - the device used to measure the quantity of heat. Heat capacity (C) - the quantity of heat required to change the temperature of the system by 1 ºC. C = q / ∆T (units are joules/ ºC) Molar heat capacity - the heat capacity of one mole of a substance Specific heat capacity • • • • • the quantity of heat required to change the temperature of one gram of a substance by 1 ºC. Sp. heat = C/ m (heat capacity/mass) Water has a relatively high specific heat. 1 calorie = the quantity of heat required to raise the temperature of 1 gram of water from 14.5 ºC to 15.5 ºC. (1 cal = 4.184 J) Specific heat varies with temperature q = m C ∆T (∆T = T final - T initial) Measuring specific heat • Simple calorimeters can be used to determine the specific heat of an insoluble solid. 1. A measured mass of water is placed in the calorimeter and the temperature taken. 2. A measured mass of the solid is heated to a measured temperature then dropped into the water. 3. Heat transfers from the solid to the water - the water temperature rises and the solid temperature falls until they are equal. 4. The final temperature of the water is taken. 5. All the heat lost by the solid is gained by the water in the calorimeter. 6. The quantity of heat transferred can be determined from the mass and temperature change of the water. For constant pressure calorimetry, ∆H = -qcalorim Bomb Calorimetry (constant volume): ∆U = -qcalorim Hess's Law of Constant Heat Summation: • The heat of a reaction is constant, whether the reaction is carried out directly in one step or indirectly through a number of steps. Standard Enthalpies of Formation: Standard state for solids and liquids - the pure element or compound at 1 atm and the temperature of interest. Standard state for a gas - the gas behaving as an ideal gas at 1 atm and the temperature of interest. Standard enthalpy of reaction (∆Hº)- the enthalpy change for a reaction where the reactants in their standard states yield products in their standard states. Standard enthalpy of formation (∆Hºf) - the enthalpy change that occurs in the formation of 1 mole of the substance from its elements when all substances are in their standard states. (Also called the heat of formation). • • The elements must be in their reference forms (in their most stable form at 1 atm and the given temp). The standard enthalpy of formation of a pure element in its reference form is 0. Calculations: ∆Hºreaction = Σ ∆Hºf(products) - Σ ∆Hºf(reactants)