Thermodynamics
... •property of a system not determined by its history •depends only on its present condition •E is a state function Path: •depends on manner in which process is carried out •q and w are path functions ...
... •property of a system not determined by its history •depends only on its present condition •E is a state function Path: •depends on manner in which process is carried out •q and w are path functions ...
Please use your NUMERICAL RESPONSE SHEET to answer the
... Use the following information to answer the following question. In an experiment, Nicole and Erik add 40 g of lead(II) nitrate to 36 g of sodium iodide. They use a 150 mL beaker having a mass of 100 g for the reaction and a measuring scale to find the mass of the reactants and products. ...
... Use the following information to answer the following question. In an experiment, Nicole and Erik add 40 g of lead(II) nitrate to 36 g of sodium iodide. They use a 150 mL beaker having a mass of 100 g for the reaction and a measuring scale to find the mass of the reactants and products. ...
Bio Boot Camp - Tredyffrin/Easttown School District
... Water • The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. This ...
... Water • The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface. This ...
Chemistry of Life
... Hydrogen Bonds • A bond between a hydrogen molecule with a ________ positive charge and another atom or molecule with a partial or __________ negative charge. ...
... Hydrogen Bonds • A bond between a hydrogen molecule with a ________ positive charge and another atom or molecule with a partial or __________ negative charge. ...
Chapter 3. Analysis of Environmental System 3.1 Analysis of a
... Eq.(3.1.3) may be solved without any difficulty if we follow the process as we have done so far. Especially, when discharging rate of a pollutant into the lake is constant, it is much easier because the condition is steady state, dC/dt = 0. ...
... Eq.(3.1.3) may be solved without any difficulty if we follow the process as we have done so far. Especially, when discharging rate of a pollutant into the lake is constant, it is much easier because the condition is steady state, dC/dt = 0. ...
CHEM 13 NEWS EXAM 1998 - University of Waterloo
... 22. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 grams of hydrogen and the other holds 8.0 grams of oxygen. Which one of the following statements regarding these gas samples is false? (The relative atomic mass of oxygen is 16.0 and that of hydrogen is 1. ...
... 22. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 grams of hydrogen and the other holds 8.0 grams of oxygen. Which one of the following statements regarding these gas samples is false? (The relative atomic mass of oxygen is 16.0 and that of hydrogen is 1. ...
11 Thermodynamics 9 26 05
... of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
... of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
Describing Matter
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
... observed without changing a substance into another. - Chemical properties can be observed only by changing substances into other substances. * The scissors are physically hard, they chemically rusted. 4. A metal melts at 450 degrees C. Is this a physical or chemical property – explain? - Melting is ...
snc 2do unit: chemistry unit test review questions
... 8. Describe three tests you can perform to check if an unknown substance is an acid or a base? 9. What type of substances antacids and how can they be useful to us? 10. Classify each of the following substances as an acid, base or neutral: a) HCl(aq) b) NaOH (aq) c) NaCl d) H2SO4 (aq) 11. Which of t ...
... 8. Describe three tests you can perform to check if an unknown substance is an acid or a base? 9. What type of substances antacids and how can they be useful to us? 10. Classify each of the following substances as an acid, base or neutral: a) HCl(aq) b) NaOH (aq) c) NaCl d) H2SO4 (aq) 11. Which of t ...
H 2 and H 2 + O 2 g H 2 O and H 2 O Hydrogen + Oxygen g Water
... of reactants and products (remember, we want to see if there was any change in mass)! Measure out 50ml of hydrochloric acid (HCl) into a beaker (find its mass) Measure out 50ml of copper sulfate (CuSO4) into a beaker (find its mass) Mix the two liquids – what is the final mass Write a word and ...
... of reactants and products (remember, we want to see if there was any change in mass)! Measure out 50ml of hydrochloric acid (HCl) into a beaker (find its mass) Measure out 50ml of copper sulfate (CuSO4) into a beaker (find its mass) Mix the two liquids – what is the final mass Write a word and ...
Carbon-12 Stable
... How do we distinguish one example of matter from another? Physical Properties of Matter A Physical Property is something that can be observed or measured without changing the matter’s identity. -They help you identify a substance Examples: Conductivity- ability to transfer heat or electricity State ...
... How do we distinguish one example of matter from another? Physical Properties of Matter A Physical Property is something that can be observed or measured without changing the matter’s identity. -They help you identify a substance Examples: Conductivity- ability to transfer heat or electricity State ...
Document
... of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
... of exergonic reactions that power the work of the cell. The product of each reaction becomes the reactant for the next, so no reaction reaches equilibrium. ...
Thermodynamic Concep..
... Biochemistry, thank goodness, occurs at constant temperature and pressure (this just saved us many pages of grief). If the pressure is held constant, we have another name for the heat generated or consumed by the system. We call it the enthalpy change and give it the symbol “H”. Thus we can write a ...
... Biochemistry, thank goodness, occurs at constant temperature and pressure (this just saved us many pages of grief). If the pressure is held constant, we have another name for the heat generated or consumed by the system. We call it the enthalpy change and give it the symbol “H”. Thus we can write a ...
In Class Overview of Chapter
... Please take some time to review state functions, the first law of thermodynamics, enthalpy and internal energy. In this chapter we will learn what determines the extent of a reaction. Thermodynamics is a powerful tool in chemistry, physics and engineering. In chapter 14, we learned about how fast re ...
... Please take some time to review state functions, the first law of thermodynamics, enthalpy and internal energy. In this chapter we will learn what determines the extent of a reaction. Thermodynamics is a powerful tool in chemistry, physics and engineering. In chapter 14, we learned about how fast re ...
Year 8 Science Assessment Point 2
... 1. Reversible reaction: A reaction that can go backwards and the products be converted back into the reactants 2. Exothermic reaction: A reaction that releases heat so feels hot 3. Endothermic reaction: A reaction that takes in heat from its surroundings to feels cold ...
... 1. Reversible reaction: A reaction that can go backwards and the products be converted back into the reactants 2. Exothermic reaction: A reaction that releases heat so feels hot 3. Endothermic reaction: A reaction that takes in heat from its surroundings to feels cold ...
Lecture 5 - Chemistry Courses
... System is like a bank, where currencies can be deposited or withdrawn hereUm(0)is the internal molar energy atT=0 (no translation) - so energy can also come from internal structure of atoms. If a gas has polyatomic molecules, they can rotate about three axes as well, for an additional energy contrib ...
... System is like a bank, where currencies can be deposited or withdrawn hereUm(0)is the internal molar energy atT=0 (no translation) - so energy can also come from internal structure of atoms. If a gas has polyatomic molecules, they can rotate about three axes as well, for an additional energy contrib ...
Introduction to Thermodynamics
... is considered to be the chief contribution of the nineteenth century to physical science. In the past, there was some belief in conservation of matter (except for combustion), and Lavoisier demonstrated this in chemical reactions. ...
... is considered to be the chief contribution of the nineteenth century to physical science. In the past, there was some belief in conservation of matter (except for combustion), and Lavoisier demonstrated this in chemical reactions. ...
Enzymes
... The active site places substrates in the correct orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive microenvironment for a specific re ...
... The active site places substrates in the correct orientation for the reaction. As the active site binds the substrate, it may put stress on bonds that must be broken, making it easier to reach the transition state. R groups at the active site may create a conducive microenvironment for a specific re ...
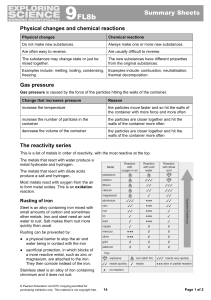
9F Reactivity - Parrs Wood High School
... ● The metals lower in the reactivity series are easier to extract from their ores and they have been available to use as the pure elements for much longer. ● Metals from zinc downwards in the reactivity series can be extracted from their ores by heating with carbon. ● Metals above zinc in the reacti ...
... ● The metals lower in the reactivity series are easier to extract from their ores and they have been available to use as the pure elements for much longer. ● Metals from zinc downwards in the reactivity series can be extracted from their ores by heating with carbon. ● Metals above zinc in the reacti ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.