Chemistry - Edgbarrow School
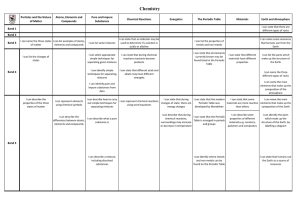
... I can name the main I can represent chemical reactions out simple techniques for changes of state, there are Periodic Table was materials are more reactive elements that make up the using word equations separating mixtures energy changes developed by Mendeleev than others composition of the Earth ...
... I can name the main I can represent chemical reactions out simple techniques for changes of state, there are Periodic Table was materials are more reactive elements that make up the using word equations separating mixtures energy changes developed by Mendeleev than others composition of the Earth ...
Chapter 3 – part I Sections 1-3
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
... • What is oxidized and reduced are always reactants, the products are the result of the redox. • So if asked “what is ox or red?”, answer is reactant ...
Reaction Rates
... The rate of reaction refers to the amount of reactant present at the start of the reaction the speed of the reaction the amount of product produced from the reaction How concentrated the reactants are ...
... The rate of reaction refers to the amount of reactant present at the start of the reaction the speed of the reaction the amount of product produced from the reaction How concentrated the reactants are ...
2011-2012 Summer Packet - Tenafly Public Schools
... These include phase changes, warming, tearing, grinding, and dissolving. Chemical changes always alter the chemical formula of a substance. Usually chemical changes produce a color change, a gas or a precipitate. Chemical changes include burning, oxidizing, corroding, exploding, reaction with acid a ...
... These include phase changes, warming, tearing, grinding, and dissolving. Chemical changes always alter the chemical formula of a substance. Usually chemical changes produce a color change, a gas or a precipitate. Chemical changes include burning, oxidizing, corroding, exploding, reaction with acid a ...
Reaction Systems Engineering II (part 1)
... rH° = (–230.0) – (–285.8) = 55.8 kJ mol–1. rS° = –10.8 – 69.9 = –80.7 J K–1 mol–1. rG° = rH° – TrS° = 55.8 – 298(–80.7) / 1000 = 79.85 kJ mol–1 Kw = exp(–rG° / RT) = exp[–79.851000 / (8.3145298)] = 1.0110–14 mol2 kg–2 2) rG° = rH° – TrS° = 55.8 – 348(–80.7) / 1000 = 83.88 kJ mol–1 Kw = ...
... rH° = (–230.0) – (–285.8) = 55.8 kJ mol–1. rS° = –10.8 – 69.9 = –80.7 J K–1 mol–1. rG° = rH° – TrS° = 55.8 – 298(–80.7) / 1000 = 79.85 kJ mol–1 Kw = exp(–rG° / RT) = exp[–79.851000 / (8.3145298)] = 1.0110–14 mol2 kg–2 2) rG° = rH° – TrS° = 55.8 – 348(–80.7) / 1000 = 83.88 kJ mol–1 Kw = ...
Thermochemistry - Moorpark College
... potential energy due to the structure of the atoms, the attachment between atoms, the atoms’ positions relative to each other in the molecule, or the molecules, relative positions in the structure Laws of Thermodynamics: Laws of thermodynamics are useful in predicting outcomes, but unlike a theory d ...
... potential energy due to the structure of the atoms, the attachment between atoms, the atoms’ positions relative to each other in the molecule, or the molecules, relative positions in the structure Laws of Thermodynamics: Laws of thermodynamics are useful in predicting outcomes, but unlike a theory d ...
AP Thermo I Notes
... ∆Hcomb-heat of combustion-combusting a substance in oxygen ∆Hf-heat of formation-heat associated with the formation of a compound from its elements. This one is kind of important. Since the amount of enthalpy change depends on temp., pressure, and state (phase), it helps to compare reactions a ...
... ∆Hcomb-heat of combustion-combusting a substance in oxygen ∆Hf-heat of formation-heat associated with the formation of a compound from its elements. This one is kind of important. Since the amount of enthalpy change depends on temp., pressure, and state (phase), it helps to compare reactions a ...
Entropy (Part I)
... also predict that it would be close to zero, since the number of moles of gaseous molecules did not change from reactant to product. ...
... also predict that it would be close to zero, since the number of moles of gaseous molecules did not change from reactant to product. ...
CHAPTER 1, MATTER AND CHANGE Section 1, Chemistry Is a
... A chemical property relates to a substance’s ability undergo changes that transform it into different substances. A chemical change or chemical reaction is a change in which one or more substances are converted into different substances. The reactant is the substance(s) that reacts in a chemical cha ...
... A chemical property relates to a substance’s ability undergo changes that transform it into different substances. A chemical change or chemical reaction is a change in which one or more substances are converted into different substances. The reactant is the substance(s) that reacts in a chemical cha ...
4 Properties of Matter Chapter Outline Properties of Substances
... Energy can be converted from one form to another. In chemistry, energy is most frequently released as heat. © 2014 John Wiley & Sons, Inc. All rights reserved. ...
... Energy can be converted from one form to another. In chemistry, energy is most frequently released as heat. © 2014 John Wiley & Sons, Inc. All rights reserved. ...
Lab 1-1 - My eCoach
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
... INTRODUCTION: Chemistry is a science that investigates changes in matter. Chemical reactions are the changes matter undergoes. The changes you can observe are called “macroscopic changes.” Often these changes, such as color changes, the formation of a solid (precipitation), or the formation of gas b ...
Chemistry 101 2007
... cannot?). e.g. H2 and O2 combine with each other to form water, H2O. The properties of water are very different from those of H2 and O2. ...
... cannot?). e.g. H2 and O2 combine with each other to form water, H2O. The properties of water are very different from those of H2 and O2. ...
Matter - Clayton State University
... - change of state (from liquid to gas, from liquid to solid, etc.) - when liquid water evaporates to steam or freezes to ice ...
... - change of state (from liquid to gas, from liquid to solid, etc.) - when liquid water evaporates to steam or freezes to ice ...
Predicting Products online assistance #3
... 1. synthesis - two reactants combine to form one product 2. decomposition - one reactant decomposes, or breaks apart, into two or more products. 3. single replacement - an element replaces another in a compound. 4. double replacement - the elements in two compounds switch partners to form two new co ...
... 1. synthesis - two reactants combine to form one product 2. decomposition - one reactant decomposes, or breaks apart, into two or more products. 3. single replacement - an element replaces another in a compound. 4. double replacement - the elements in two compounds switch partners to form two new co ...
15-2 Thermodynamic Processes and the First Law
... coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. ...
... coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. ...
States of Matter Web
... Go to www.kentchemistry.com/links/Matter/PhysicalChemicalChanges.htm and answer the following questions: What is the difference between a physical change and a chemical change? ...
... Go to www.kentchemistry.com/links/Matter/PhysicalChemicalChanges.htm and answer the following questions: What is the difference between a physical change and a chemical change? ...
Enthalpy
... reaction that forms 1 mol of the compound from its elements, with all substances in their standard states. The most stable form of the element is used. E.g. O2 not O3, C(graphite not diamond) The standard enthalpy of formation of the most stable form of any element is zero. Using Enthalpies of Forma ...
... reaction that forms 1 mol of the compound from its elements, with all substances in their standard states. The most stable form of the element is used. E.g. O2 not O3, C(graphite not diamond) The standard enthalpy of formation of the most stable form of any element is zero. Using Enthalpies of Forma ...
AP_PPT_ch_17
... LO 5.13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both Ho and So, and calculation or estimation of Go when needed. LO 5.14 The student is able to determine whethe ...
... LO 5.13 The student is able to predict whether or not a physical or chemical process is thermodynamically favored by determination of (either quantitatively or qualitatively) the signs of both Ho and So, and calculation or estimation of Go when needed. LO 5.14 The student is able to determine whethe ...
work
... It is an experimental fact that the work done during these two different processes is the same. The total work is the same in all adiabatic processes between any two equilibrium states having the same kinetic and potential energy!!! This is ...
... It is an experimental fact that the work done during these two different processes is the same. The total work is the same in all adiabatic processes between any two equilibrium states having the same kinetic and potential energy!!! This is ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.























