Chapter 8 - Clayton State University
... Also known as Law of Conservation of Energy The total amount of energy in the universe is constant. ...
... Also known as Law of Conservation of Energy The total amount of energy in the universe is constant. ...
Test 1 - UTC.edu
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
... 14. Which one of the following statements about atoms and subatomic particles is correct? A) The proton and the neutron have identical masses. B) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons C) The neutron's mass is equal to that of a proton plus an electron. D) An ...
Chemical Equations TrackStar Assignment
... 1. What does the Law of Conservation of Mass state? 2. What is a reversible reaction and how is it indicated? 3. Write the reaction for a silver spoon tarnishing. What type of reaction is this? 4. Write the reaction for the burning of Methane gas (the gas used in Chemistry lab). What type of reactio ...
... 1. What does the Law of Conservation of Mass state? 2. What is a reversible reaction and how is it indicated? 3. Write the reaction for a silver spoon tarnishing. What type of reaction is this? 4. Write the reaction for the burning of Methane gas (the gas used in Chemistry lab). What type of reactio ...
File
... In a physical change, the substance involved remains the same. The substance may change form or state, however. All changes of state are physical changes. There are other physical changes that are not changes of state. Dissolving is a physical change. When sugar is dissolved it spreads out in the wa ...
... In a physical change, the substance involved remains the same. The substance may change form or state, however. All changes of state are physical changes. There are other physical changes that are not changes of state. Dissolving is a physical change. When sugar is dissolved it spreads out in the wa ...
+ H 2
... • Consider the following reactions of three unknown metals X, Y and Z. 2XNO3(aq) + Y(s) → 2X(s) + Y(NO3)2(aq) Y(NO3)2(aq) + Z(s) → No reaction 2XNO3(aq) + Z(s) → 2X(s) + Z(NO3)2(aq) What is the order of increasing reactivity of the metals (least reactive first)? X < Z < Y ...
... • Consider the following reactions of three unknown metals X, Y and Z. 2XNO3(aq) + Y(s) → 2X(s) + Y(NO3)2(aq) Y(NO3)2(aq) + Z(s) → No reaction 2XNO3(aq) + Z(s) → 2X(s) + Z(NO3)2(aq) What is the order of increasing reactivity of the metals (least reactive first)? X < Z < Y ...
The first law of thermodynamics
... pressure of 2.0 atm from 10.0 L to 2.0 L (B to C). In this process, some heat flows out of the gas and the temperature drops. Heat is then added to the gas (C to A), holding the volume constant, and the pressure and temperature are allowed to rise until the temperature reaches its original value. Sk ...
... pressure of 2.0 atm from 10.0 L to 2.0 L (B to C). In this process, some heat flows out of the gas and the temperature drops. Heat is then added to the gas (C to A), holding the volume constant, and the pressure and temperature are allowed to rise until the temperature reaches its original value. Sk ...
Chem161 Chapter 6
... Assume that the specific heat of the solution is 4.18 J/g°C, the density is 1.00 g/mL, and that the calorimeter itself absorbs a negligible amount of heat ...
... Assume that the specific heat of the solution is 4.18 J/g°C, the density is 1.00 g/mL, and that the calorimeter itself absorbs a negligible amount of heat ...
Gas and Thermo Notes
... Standard state - is a defined set of standard conditions for thermodynamic measurements to assure uniformity of data obtained from different research groups. Standard state is defined at 25 ˚C and 1 atmosphere pressure. Standard state conditions are indicated by the appearance of the character ˚ imm ...
... Standard state - is a defined set of standard conditions for thermodynamic measurements to assure uniformity of data obtained from different research groups. Standard state is defined at 25 ˚C and 1 atmosphere pressure. Standard state conditions are indicated by the appearance of the character ˚ imm ...
Chapter 2 - OrgSites.com
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
... 14. Describe the relationship between an electron’s potential energy and the distance it is from the nucleus. 15. When an electron absorbs energy, they are called “excited.” When an electron loses energy, it “falls back.” Give an example of each of these processes. ...
Lecture 3: FIRST LAW OF THERMODYNAMICS
... water back up from 0◦ to its final temperature, or refreeze some ice. This is an example of using an equivalent process to make a thermodynamic problem mathematically tractible. It is legitimate because temperature and internal energy are state variables. ...
... water back up from 0◦ to its final temperature, or refreeze some ice. This is an example of using an equivalent process to make a thermodynamic problem mathematically tractible. It is legitimate because temperature and internal energy are state variables. ...
Chemistry: The Study of Change
... • All science has shown the universe is constantly expanding, spreading out from a common origin (increasing entropy). • The Big bang theory describes at one “time” all energy/mass was found in a ultra-dense point. • There has never been a consistent theory as to why an infinitely dense mass existed ...
... • All science has shown the universe is constantly expanding, spreading out from a common origin (increasing entropy). • The Big bang theory describes at one “time” all energy/mass was found in a ultra-dense point. • There has never been a consistent theory as to why an infinitely dense mass existed ...
Chemical change is a process that involves recombining atoms and
... Predict the products of formation (synthesis) and decomposition, single and double replacement and hydrocarbon combustion chemical reactions, when given the reactants Interpret balanced chemical equations in terms of moles of chemical species and relate the mole concept to the law of conservation of ...
... Predict the products of formation (synthesis) and decomposition, single and double replacement and hydrocarbon combustion chemical reactions, when given the reactants Interpret balanced chemical equations in terms of moles of chemical species and relate the mole concept to the law of conservation of ...
Changes in Matter: Physical and Chemical Changes
... Potential - stored energy in molecules waiting to be changed Thermal – heat that changes state of matter (physical). Heat makes molecules move faster, causing them to bump into each other and created new molecules(chemical) Chemical – molecules combine to create new molecules Electromagnetic – sun p ...
... Potential - stored energy in molecules waiting to be changed Thermal – heat that changes state of matter (physical). Heat makes molecules move faster, causing them to bump into each other and created new molecules(chemical) Chemical – molecules combine to create new molecules Electromagnetic – sun p ...
PHYSICAL PROPERTIES - can observe w/o changing the
... 5 Signs of a Chemical Reaction: Spontaneous color change Production of a new substance (solid = precipitation, gas = bubbling) Formation of a new odor Spontaneous change in temperature Spontaneous generation of light ...
... 5 Signs of a Chemical Reaction: Spontaneous color change Production of a new substance (solid = precipitation, gas = bubbling) Formation of a new odor Spontaneous change in temperature Spontaneous generation of light ...
Document
... A. They have shorter wavelengths than X-rays. B. They give off more energy than gamma rays. C. They have longer wavelengths than ultraviolet waves. D. They have higher frequencies than visible light waves. 45. If 15 N of force are applied to a cart to move it a distance of 5 m, how much work is done ...
... A. They have shorter wavelengths than X-rays. B. They give off more energy than gamma rays. C. They have longer wavelengths than ultraviolet waves. D. They have higher frequencies than visible light waves. 45. If 15 N of force are applied to a cart to move it a distance of 5 m, how much work is done ...
Lesson 1 of 6
... • In any chemical reaction, mass is conserved. – In other words, the mass of the reactant(s) is the same as the mass of the product(s). – The elements on one side of the equation are the same as those on the other. – Matter cannot be created nor destroyed. ...
... • In any chemical reaction, mass is conserved. – In other words, the mass of the reactant(s) is the same as the mass of the product(s). – The elements on one side of the equation are the same as those on the other. – Matter cannot be created nor destroyed. ...
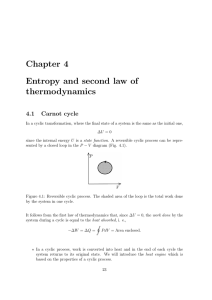
Chapter 4 Entropy and second law of thermodynamics
... CHAPTER 4. ENTROPY AND SECOND LAW OF THERMODYNAMICS 6. Since entropy is proportional to the ”multiplicity of a macrostate”, it can be viewed as a ”measure of disorder”: ...
... CHAPTER 4. ENTROPY AND SECOND LAW OF THERMODYNAMICS 6. Since entropy is proportional to the ”multiplicity of a macrostate”, it can be viewed as a ”measure of disorder”: ...
Chapter 6
... • The same amount of energy leaving a system will enter the surroundings (or vice versa), so the total amount of energy remains constant. ...
... • The same amount of energy leaving a system will enter the surroundings (or vice versa), so the total amount of energy remains constant. ...
Application , First, Law of Thermodynamics
... 1. As an assessment tool, have the learners answer the following questions and place it on a ¼ sheet of paper: a. Given the following processes: i. Expansion of the burned gasoline–air mixture in the cylinder of an automobile engine ii. Opening a bottle of champagne iii. Filling a scuba tank with co ...
... 1. As an assessment tool, have the learners answer the following questions and place it on a ¼ sheet of paper: a. Given the following processes: i. Expansion of the burned gasoline–air mixture in the cylinder of an automobile engine ii. Opening a bottle of champagne iii. Filling a scuba tank with co ...
Thermodynamics lesson 1 Tempersture
... it is something for a material and NOT an object. Work out k for Copper and you can do the sums for any object made of copper • The gradient: A driving force behind so much of things happening in physics. ΔT here but ...
... it is something for a material and NOT an object. Work out k for Copper and you can do the sums for any object made of copper • The gradient: A driving force behind so much of things happening in physics. ΔT here but ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.