* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chem161 Chapter 6
Water splitting wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Solar air conditioning wikipedia , lookup
Marcus theory wikipedia , lookup
Stoichiometry wikipedia , lookup
Thermodynamics wikipedia , lookup
Electrolysis of water wikipedia , lookup
Heat transfer wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
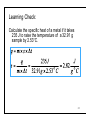
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work • Energy is the ability to do work (move mass over a distance) or transfer heat • Types: kinetic and potential • kinetic: the energy of motion • potential: the stored energy in matter • Internal energy (E): the sum of the kinetic and potential energy for each particle in the system 2 Kinetic Energy: The Energy Of Motion • KE=½mv2 • Energy can be transferred by moving particles • Collision of fast particles with slower particles causes the slow particle to speed up while the fast molecule slows • this is why hot water cools in contact with cool air 3 Potential Energy Depends on Position • Potential energy increases when: objects that attract move apart, or objects that repel move toward each other • Stored energy that can be converted to kinetic energy 4 Your Turn! Which of the following is not a form of kinetic energy? A. A pencil rolls across a desk B. A pencil is sharpened C. A pencil is heated D. All are forms of kinetic energy E. None are forms of kinetic energy 5 Law Of Conservation Of Energy • Energy cannot be created or destroyed but can be transformed from one form of energy to another • Also known as the first law of thermodynamics • How does water falling over a waterfall demonstrate this law? 6 Problems: 3, 5, 9 7 Heat And Temperature Are Not The Same • The temperature of an object is proportional to the average kinetic energy of its particles —the higher the average kinetic energy, the higher the temperature • Heat is energy (also called thermal energy) transferred between objects caused by differences in their temperatures until they reach thermal equilibrium 8 Units Of Energy • SI unit is the Joule, J • J = kg·m/s2 • If the calculated value is greater than 1000 J, use the kJ • British unit is the calorie, cal • cal = 4.184 J (exact) • Nutritional unit is the Calorie (note capital c), which is one kilocalorie • 1 Cal = 1 kcal = 4.184 kJ 9 Internal Energy is Conserved • 1st Law of Thermodynamics: For an isolated system the internal energy (E) is constant: Δ E = Ef - E i = 0 Δ E = Eproduct - Ereactant = 0 • We canʼt measure the internal energy of anything, so we measure the changes in energy • E is a state function • E = work + heat 10 What Is Temperature? • Temperature (T) is proportional to the average kinetic energy of all particle units: °C, °F, K • Avg KE= ½ mvavg2 • At a high temperature, most molecules are moving at higher average 11 State Function • A property whose value depends only on the present state of the system, not on the method or mechanism used to arrive at that state • Position is a state function: both train and car travel to the same locations although their paths vary • The actual distance traveled does vary with path New York Los Angeles 12 Problems: 41, 43 13 Heat Transfer, q • Heat (q) = the transfer of energy from regions of high temperature to regions of lower temperature • units: J, cal, kg·m2/s2 • A calorie is the amount of energy needed to raise the temperature of 1.00 g water from 14.5 to 15.5 °C • A metal spoon at 25°C is placed in boiling water. What happens? 14 Surroundings / System / Universe • System: the reaction or area under study • Surroundings: the rest of the universe • Open systems can gain or lose mass and energy across their boundaries • i.e. the human body • Closed systems can absorb or release energy, but not mass, across the boundary • i.e. a light bulb • Isolated systems cannot exchange matter or energy with their surroundings Adiabatic • i.e. a stoppered Thermos bottle 15 The Sign Convention • Endothermic systems require energy to be added to the system, thus the q is (+) • Exothermic reactions release energy to the surroundings. Their q is (-) • Energy changes are measured from the point of view of the system 16 Your Turn! A cast iron skillet is moved from a hot oven to a sink full of water. Which of the following is not true? A. The water heats B. The skillet cools C. The heat transfer for the skillet has a (-) sign D. The heat transfer for the skillet is the same as the heat transfer for the water E. None of these are untrue 17 Heat Capacity and Transfer • Heat capacity (C)- the (intensive) ability of an object with constant mass to absorb heat. • a/k/a calorimeter constant • Varies with the sample mass and the identity of the substance • Units: J/°C • q=C×Δt • q= heat transferred • C= heat capacity of object • Δt= Change in Temperature (tfinal-tinitial) 18 Learning Check: A cup of water is used in an experiment. Its heat capacity is known to be 720 J/ °C. How much heat will it absorb if the experimental temperature changed from 19.2 °C to 23.5 °C? 19 Heat Transfer and Specific Heat • Specific heat (s)- The (extensive) ability of a substance to store heat. • C = m×s • Units : J/g·°C; J/g · K; J/mol · K • q=m×Δt×s • q= heat transferred • m= mass of object • Δt= Change in Temperature (tfinal-tinitial) 20 Specific Heats • Substances with high specific heats resist temperature changes • Note that water has a very high specific heat • (this is why coastal temperatures are different from inland temperatures) Substance Specific Heat, J/ g °C (25 °C) Carbon (graphite) 0.711 Copper 0.387 Ethyl alcohol 2.45 Gold 0.129 Granite 0.803 Iron 0.4498 Lead 0.128 Olive oil 2.0 Silver 0.235 Water (liquid) 4.18 21 Learning Check: Calculate the specific heat of a metal if it takes 235 J to raise the temperature of a 32.91 g sample by 2.53°C. 22 Problems: 45, 47, 49 23 The First Law Of Thermodynamics Explains Heat Transfer • If we monitor the heat transfers (q) of all materials involved and all work processes, we can predict that their sum will be zero • By monitoring the surroundings, we can predict what is happening to our system • Heat transfers until thermal equilibrium, thus the final temperature is the same for all materials 24 Learning Check: A 43.29 g sample of solid is transferred from boiling water (T=99.8°C) to 152 g water at 22.5°C in a coffee cup. The Twater rose to 24.3°C. Calculate the specific heat of the solid. qsample+ qwater + qcup=0 qcup is neglected in problem qsample = - qwater 25 Your Turn! What is the heat capacity of the container if 100.g of water (s=4.184 J/g·°C) at 100.°C are added to 100. g of water at 25°C in the container and the final temperature is 61°C? A. 870 J/°C B. 35 J/°C C. -35 J/°C D. -870 J/°C E. None of these 26 Chemical Potential Energy • Chemical bond: net attractive forces that bind atomic nuclei and electrons together • Exothermic reactions form stronger bonds in the product than in the reactant and release energy (-E) • Endothermic reactions break stronger bonds than they make and require energy (+E) 27 Work and Pistons • Pressure = force/ area • If the container volume changes, the pressure changes • Work = -P×ΔV • units: L • atm • 1 L • atm = 101 J • In expansion, ΔV>0, and is exothermic • Work is done by the system in expansion 28 How does work relate to reactions? • Work = Force · Distance • is most often due to the expansion or contraction of a system due to changing moles of gas. • Gases push against the atmospheric pressure , so Psystem = -Patm • w = -Patm×ΔV • The deployment of an airbag is one example of this process. 1 C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) 6 moles of gas → 7 moles of gas 29 Learning Check: P-V work • Ethyl chloride is prepared by reaction of ethylene with HCl. How much PV work (in J) is done if 89.5 g ethylene and 125 g of HCl are allowed to react at atmospheric pressure and the volume change is -71.5 L? w = -1atm × -71.5L =71.5 L·atm w = 0.708 J • Calculate the work (in kilojoules) done during a synthesis of ammonia in which the volume contracts from 8.6 L to 4.3L at a constant external pressure of 44 atm w = -44atm × (4.3-8.6)L =189.2L·atm w = 0.0019 kJ 30 Energy Can Be Transferred as Heat and Work • ΔE= q + w • internal energy changes are state functions 31 Your Turn! When TNT is combusted in air, it is according to the following reaction: 4C6H2(NO2)3CH3(s) + 17 O2(g) →24 CO2(g) + 10H2O(l) + 6N2(g) The reaction will do work for all of these reasons except: A. The moles of gas increase B. The volume of gas increases C. The pressure of the gas increases D. None of these 32 Problems: 23, 27 33 Calorimetry Is Used To Measure Heats Of Reaction • Heat of reaction: the amount of heat absorbed or released in a chemical reaction • Calorimeter: an apparatus used to measure temperature changes in materials surrounding a reaction that result from a chemical reaction • From the temperature changes we can calculate the heat of the reaction, q • qv; heat measured under constant volume conditions • qp: heat measured under constant pressure conditions 34 Internal Energy Is Measured With A Bomb Calorimeter • Used for reactions in which there is change in the number of moles of gas present • Measures qv • Immovable walls mean that work is zero • ΔE = qv 35 Learning Check: Bomb Calorimeter 500. mg naphthalene (C10H8) is combusted in a bomb calorimeter containing 1000. g of water. The temperature of the water increases from 20.00°C to 24.37°C. The calorimeter constant is 420 J/°C. What is the change in internal energy for the reaction? swater = 4.184 J/g°C qv reaction + qwater + qcal = 0 by the first law qwater= 1000. g ×(24.37-20.00)°C× 4.184 J/g°C qcal= 420 J/g ×(24.37-20.00)°C qv = ΔE = -2.0×104 J 36 Enthalpy Of Combustion • When one mole of a fuel substance is reacted with elemental oxygen, a combustion reaction can be written whose enthalpy is ΔΗc˚ • Is always negative • Learning Check: What is the equation associated with the enthalpy of combustion of C6H12O6(s)? C6H12O6(s) + 9O2(g) →6CO2(g) + 6H2O(l) 37 Your Turn! 252 mg of benzoic acid, C6H5CO2H, is combusted in a bomb calorimeter containing 814 g water at 20.00ºC. The reaction increases the temperature of the water to 21.70. What is the internal energy released by the process? A. B. C. D. E. -711 J -2.85 J +711 J +2.85 J None of these swater = 4.184 J/g°C 38 Enthalpy Change (ΔH) • enthalpy is the heat transferred at constant pressure • ΔH = qp • ΔE = qp - P ΔV = ΔH – PΔV • ΔH = ΔHfinal - ΔHinitial • ΔH= ΔHproduct- ΔHreactant 39 Enthalpy Measured in a Coffee Cup Calorimeter • when no change in moles of gas is expected, we may use a coffee cup calorimeter • the open system allows the pressure to remain constant • thus we measure qp • ΔE=q + w • since there is no change in the moles of gas present, there is no work • thus we also are measuring ΔE 40 Learning Check: Coffee Cup Calorimetery When 50.0 mL of .987 M H2SO4 is added to 50.0 mL of 1.00 M NaOH at 25.0 °C in a calorimeter, the temperature of the aqueous solution increases to 31.7 °C. Calculate heat for the reaction per mole of limiting reactant. Assume that the specific heat of the solution is 4.18 J/g°C, the density is 1.00 g/mL, and that the calorimeter itself absorbs a negligible amount of heat moles H2SO4 = 0.04935 moles NaOH = 0.0500, is limiting qv rxn + qcal + qsoln = 0 qsoln = 100.gsoln×(31.7-25.0)°C ×4.18J/g°C qv rxn = -2.8×103 J qv rxn = -5.6×104 J H2SO4(aq) + 2 NaOH(aq) → 2H2O(l) + Na2SO4(aq) 41 Your Turn! A sample of 50.00mL of 0.125M HCl at 22.36 ºC is added to a 50.00mL of 0.125M Ca(OH)2 at 22.36 ºC. The calorimeter constant was 72 J/g ºC. The temperature of the solution (s=4.184 J/g ºC, d=1.00 g/mL) climbed to 23.30 ºC. Which of the following is not true? A. qczl=67.9 J B. qsolution= 393.3J C. qrxn = 461.0 J D. qrxn = -461.0 J E. None of these 42 Calorimetry Overview • The equipment used depends on the reaction type. • If there will be no change in the moles of gas , we may use a coffee-cup calorimeter or a closed system. Under these circumstances, we measure qp. • If there is a large change in the moles of gas, we use a bomb calorimeter to measure qv. 43 Problems: 51, 53, 55 44 Thermochemical Equations • Relate the energy of a reaction to the quantities involved • Must be balanced, but may use fractional coefficients • quantities are presumed to be in moles 45 Learning Check: • 2C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g) ΔE = -2511 kJ • The reactants (acetylene and oxygen) have 2511 kJ more energy than the products • How many KJ are released for 1 mol C2H2? 1,256 kJ 46 Learning Check: • 6CO2(g) + 6H2O(l) → C6H12O6(s) + 6O2(g) Δ H = 2816 kJ • How many kJ are required for 44 g CO2 (MM=44.00986 g/mol)? 470 kJ • If 100. kJ are provided, what mass of CO2 can be converted to glucose? 9.38 g 47 Learning Check: Calorimetry of Chemical Reactions The meals-ready-to-eat (MRE) in the military can be heated on a flameless heater. Assume the reaction in the heater is Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g) ΔH = -353kJ What quantity of magnesium is needed to supply the heat required to warm 25 mL of water from 25 to 85 °C? Specific heat of water = 4.179 J/g °C. Assume the density of the solution is the same as for water at 25°C, 1.00 g/mL masssoln = 25 mL×1.00g/mL= 25 g qsoln = 25 g ×(85-25) °C ×4.179 J/g °C= 6.269 ×103 J massMg =0.43 g 48 Your Turn! Consider the thermite reaction. The reaction is initiated by the heat released from a fuse or reaction The enthalpy change is –852 kJ/mol Fe2O3 at 298K. 2 Al(s) + Fe2O3(s) →2Fe(s) + Al2O3(s) What mass of Fe (MM=55.847 g/mol) is made when 500. kJ are released? A. 65.5 g B. 0.587 g C. 32.8 g D. None of these 49 Learning Check: Ethyl Chloride Reaction Revisited • Ethyl chloride is prepared by reaction of ethylene with HCl: • C2H4(g) + HCl(g) → C2H5Cl(g) ΔH° = -72.3kJ • What is the value of ΔE if 89.5 g ethylene and 125 g of HCl are allowed to react at atmospheric pressure and the volume change is -71.5 L? mol HCl: 3.4283 mol C2H4: 3.1903, is Limiting ΔHrxn =-230.66 w = -1atm × -71.5L =71.5 kJ ΔE= -230 L·atm kJ MM ethylene=28.054 g/mol; MM HCl=36.4611 g/mol 50 Enthalpy Diagram 51 Problems: 57, 59 52 Hess’s Law • The overall enthalpy change for a reaction is equal to the sum of the enthalpy changes for individual steps in the reaction • For example: ΔH= -822.2 kJ • 2 Fe + 3/2 O2 →Fe2O3(s)& • Fe2O3(s) + 2Al(s) →Al2O3(s) + 2Fe ΔH= -852 kJ • 3/2 O2 + 2Al(s) →Al2O3(s ΔH= -822.2 kJ + -852 kJ -1674 kJ 53 Rules for Adding Thermochemical Reactions 1. When an equation is reversed—written in the opposite direction—the sign of H must also be reversed. 2. Formulas canceled from both sides of an equation must be for the substance in identical physical states. 3. If all the coefficients of an equation are multiplied or divided by the same factor, the value of H must likewise be multiplied or divided by that factor. 54 Strategy for Adding Reactions Together: 1. Choose the most complex compound in the equation (1) 2. Choose the equation (2 or 3 or…) that contains the compound 3. Write this equation down so that the compound is on the appropriate side of the equation and has an appropriate coefficient for our reaction 4. Look for the next most complex compound 55 Hess’s Law (Cont.) 5. Choose an equation that allows you to cancel intermediates and multiply by an appropriate coefficient 6. Add the reactions together and cancel like terms 7. Add the energies together, modifying the enthalpy values in the same way that you modified the equation • • if you reversed an equation, change the sign on the enthalpy if you doubled an equation, double the energy 56 Learning Check: • How can we calculate the enthalpy change for the reaction 2 H2(g) + N2(g) → N2H4(g) using these equations ? • N2H4(g) + H2(g) → 2 NH3(g) ΔH° = -187.6 kJ • 3H2(g) + N2(g) → 2NH3(g) ΔH° = -92.2 kJ • Reverse the first reaction (and change sign) • 2 NH3(g) → N2H4(g) + H2(g) ΔH° = +187.6 kJ • Add the second reaction (and add the enthalpy) N2(g) → 2NH3(g) • 3H2(g) + 2 ΔH° = -92.2 kJ • 2 NH3(g)+ 3H2(g) + N2(g) → N2H4(g) + H2(g)+ 2NH3(g) • 2 H2(g) + N2(g) → N2H4(g) (187.6-92.2)= +95.4 kJ 57 Learning Check: Calculate ΔH for 2C(s) + H2(g) → C2H2(g) using: ΔH° = -2599.2 • 2 C2H2(g) + 5O2(g) → 4 CO2(g) + 2H2O(l) ΔH° = -393.5 kJ • C(s) + O2(g) → CO2(g) ΔH° = +285.9 kJ • H2O(l) → H2(g) + ½ O2(g) 58 Your Turn! What is the energy of the following process: 6A + 9B + 3D + 1 F→2 G Given that: • C → A + 2B ∆H=20.2 kJ/mol • 2C + D →E + B ∆H=30.1 kJ/mol • 3E + F →2G ∆H=-80.1 kJ/mol A. 70.6 kJ (20.2×-6)+(30.1×3)+(-80.1) B. -29.8 kJ C. -111.0 kJ =-113.4 D. None of these kJ 59 State matters! • C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) • ΔH= -2043 kJ • C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l) • ΔH= -2219 kJ • note that there is a difference in energy because the states do not match • If H2O(l) → H2O(g) ΔH = 44 kJ/mol • 4H2O(l) → 4H2O(g) ΔH = 176 kJ/mol • -2219 +176 kJ = -2043 kJ 60 Standard State Most stable form of the pure substance at • 1 atm pressure • Stated temperature. If temperature is not specified, assume 25 °C • Solutions are 1M in concentration. • Measurements made under standard state conditions have the ° mark: ΔH° • Most ΔH values are given for the most stable form of the compound or element. 61 Determining the Most Stable State • The most stable form of a substance: • below the melting point is solid • above the boiling point is gas • between these temperatures is liquid • • • • What is the standard state of GeH4? mp -165 °C bp -88.5 °C gas What is the standard state of GeCl4? mp -49.5 °C bp 84 °C liquid 62 Problems: 61, 63, 65, 67, 69 63 Allotropes • Are substances that have more than one form in the same physical state • You should know which form is the most stable • C, P, O and S all have multiple allotropes. Which is the standard state for each? 64 Enthalpy Of Formation • Is the enthalpy change ΔH°f for the formation of 1 mole of a substance in its standard state from elements in their standard states • Note: ΔH°f = 0 for an element in its standard state • Learning Check: • What is the equation that describes the formation of CaCO3(s)? Ca(s) + C(gr) +3/2 O2(g) →CaCO3(s) 65 Calculating ΔH For Reactions Using ΔH°f ΔH°rxn = [sum of ΔH°f of all products] – [sum of ΔH°f of all reactants] • 2Fe(s) + 6H2O(l) 0 -285.8 → 2Fe(OH)3(s) + 3H2(g) -696.5 • CO2(g) + 2H2O(l) → 2O2(g) + CH4(g) -393.5 -285.8 0 -74.8 0 ΔH°f ΔH°f 66 Your Turn! What is the enthalpy for the following reaction? 2 HCH3CO2(aq) ∆Hfº -487 kJ/mol A. B. C. D. E. -756.28 kJ 756.28 kJ -1545.74 kJ 1545.74 kJ None of these + 2 OH-(aq) → 2H2O(l) + 2 C2H3O2(aq) -229.94 -285.84 -542.96 kJ/mol kJ/mol kJ/mol Problems: 71, 73, 75, 83, 85 68









































































![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-150x150.png)