* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy
Equipartition theorem wikipedia , lookup
Adiabatic process wikipedia , lookup
Thermal conduction wikipedia , lookup
Thermodynamic system wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
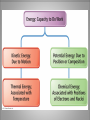
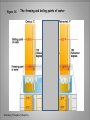
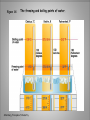
Energy A First Look at Thermodynamics… Definition… • Ability to do “work”; capacity of a system to do “work” • “Work” is defined for macroscopic systems as moving an object against a force. But chemistry deals with many microscopic systems so the physics definition of “work” does not always seem to apply. Forms… • Potential – energy due to position • Kinetic – energy due to motion • These forms of energy are interconvertible. Energy is the capacity to do work. less stable change in potential energy EQUALS kinetic energy more stable A gravitational system. The potential energy gained when a lifted weight is converted to kinetic energy as the weight falls. Energy is the capacity to do work. less stable change in potential energy EQUALS kinetic energy more stable A system of two balls attached by a spring. The potential energy gained by a stretched spring is converted to kinetic energy when the moving balls are released. Energy is the capacity to do work. less stable change in potential energy EQUALS kinetic energy more stable A system of oppositely charged particles. The potential energy gained when the charges are separated is converted to kinetic energy as the attraction pulls these charges together. Energy is the capacity to do work. less stable change in potential energy EQUALS kinetic energy more stable A system of fuel and exhaust. A fuel is higher in chemical potential energy than the exhaust. As the fuel burns, some of its potential energy is converted to the kinetic energy of the moving car. Types of Energy • There are also different types of energy: chemical electrical gravitational heat light (electromagnetic radiation) magnetic mechanical nuclear These are also interconvertible. Laws! • No matter what the conversion the system must obey the Law of Conservation of Energy. • Energy cannot be created or destroyed. (but may be converted to mass under certain conditions) Thermal Energy • In chemistry we deal primarily with thermal energy. We also encounter light and electrical energies. • Thermal energy = energy due to molecular or atomic motion • Heat (q) = transfer of chemical energy due to a temperature difference • Thermodynamics = study of heat and its transformations. • Thermochemistry = branch of thermodynamics that deals with the heat involved with chemical and physical changes. Temperature • Measure of the “hotness” or “coldness” of something • Measure of the effect of heat on an object or system • Measure of particle motion • Heat always flows from an area of higher temperature to areas of lower temperatures. Temperature Measurement • Typically measured using a thermometer which has an expandable liquid (mercury or alcohol) trapped in a sealed cylinder • US meteorological and heating unit temperatures are expressed using the Fahrenheit scale. • Water freezes at 32oF and boils at 212oF. Figure 1.8 The freezing and boiling points of water. Temperature Measurement • Other countries and the scientific community use the Celsius scale. • Water freezes at 0oC and boils at 100oC. Figure 1.8 The freezing and boiling points of water. Silberberg, Principles of Chemistry Temperature Measurement • There is a problem using either of these scales in certain systems. Because there are negative temperatures certain mathematical relationships generate impossible results. • Lord Kelvin generated a new scale with the coldest temperature as 0 K (absolute zero). The unit of temperature is a kelvin (K). The term degree is not used. • Water freezes at -273.15 K and boils at 373.15 K. Figure 1.8 The freezing and boiling points of water. Silberberg, Principles of Chemistry Temperature Conversions • oF oC o F -32 o = C 1.8 • oC K C + 273.15 = K o C x 1.8 + 32 = F o o Systems… • Changes in thermal energy of systems are determined by transfer of heat (q) observed by changes in temperature. • First you need to define what your system and surroundings are… • When heat moves out of a system to the surroundings the process is exothermic for the system. • When heat moves into a system from the surroundings the process is endothermic for the system. A system transferring energy as heat only. Exothermic Endothermic Heat Units Joule (J) 1 J = 1 kg*m2/s2 Calorie (cal) 1 cal = 4.18J British Thermal Unit 1 Btu = 1055 J Specific Heat Capacity • Substances respond differently to the same quantity of energy. • Consider a wooden spoon and a metal spoon in a pot of boiling water. Which spoon gets hotter? • Specific heat capacity (c) – energy required to raise 1 gram of substance by 1oC J/g. oC (J g-1 oC-1) or kcal/g. oC (kcal g-1 oC-1) Heat Calculations q = specific heat capacity x mass x temperature PROBLEM: PLAN: A layer of copper welded to the bottom of a skillet weighs 125 g. How much heat is needed to raise the temperature of the copper layer from 250C to 300.0C? The specific heat capacity (c) of Cu is 0.387 J/g*K. Given the mass, specific heat capacity and change in temperature, we can use q = c x mass x T to find the answer. T in 0C is the same as for K. SOLUTION: q = 0.387 J g*K 0 x 125 g x (300-25) C = 1.33x104 J Silberberg, Principles of Chemistry Enthalpy The Meaning of Enthalpy w = - PV H = E + PV where H is enthalpy H = E + PV H ≈ E in 1. Reactions that do not involve gases. 2. Reactions in which the number of moles of gas does not change. 3. Reactions in which the number of moles of gas does change but q is >>> PV. qp = E + PV = H For most of the processes we will encounter this semester we can use ΔH as heat exchanged by a system. Enthalpy ΔH for a reaction is calculated as: ΔHproducts – ΔHreactants If the ΔH is positive, energy of the products is higher than reactants and the process is endothermic. If the ΔH is negative, energy of the reactants is higher than products and the process is exothermic. Enthalpy diagrams for exothermic and endothermic processes. Enthalpy, H CH4 + 2O2 H < 0 CO2 + 2H2O A CO2(g) + 2H2O(g) H2O(l) H2O(g) Hinitial heat out Enthalpy, H CH4(g) + 2O2(g) H > 0 H2O(l) Hfinal Exothermic process B H2O(g) Hfinal heat in Hinitial Endothermic process Determining the heat exchange in a chemical change A bomb calorimeter AMOUNT (mol) of compound A Summary of the relationship between amount (mol) of substance and the heat (kJ) transferred during a reaction. AMOUNT (mol) of compound B molar ratio from balanced equation HEAT (kJ) Hrxn (kJ/mol) gained or lost Using the Heat of Reaction (Hrxn) to Find Amounts PROBLEM: The major source of aluminum in the world is bauxite (mostly aluminum oxide). Its thermal decomposition can be represented by Al2O3(s) 2Al(s) + 3/2O2(g) Hrxn = 1676 kJ If aluminum is produced this way, how many grams of aluminum can form when 1.000x103 kJ of heat is transferred? SOLUTION: 1.000x103 kJ x 2 mol Al 1676 kJ 26.98 g Al 1 mol Al = 32.20 g Al Reaction Progress • All reactions have an energy barrier that must be overcome. Activation energy (Ea) This is an exothermic reaction. Energy of reactants Energy of products Reaction Progress Activation energy (Ea) This diagram illustrates an endothermic reaction. Energy of reactants Energy of products Reaction Enthalpy The difference between the starting and ending energies is ΔH. ΔH Catalysts reduce the activation energy of a reaction. Reaction energy diagram of a catalyzed and an uncatalyzed process. Disorder aka Entropy • Systems tend to greater disorder or greater energy dispersal. • Disorder is the preferred state. • Energy dispersal or system disorder is called entropy. • All systems work to achieve lowest energy and greatest disorder or a balance between these goals.